
Concept explainers
(a)
Interpretation: To classify carbamoyl phosphate in terms of nitrogen content as
Concept introduction: Carbamoyl phosphate is an intermediate or one of the fuel for the urea cycle. Ammonium ion produced via oxidative deamination reaction is converted into the carbamoyl phosphate which then enters the urea cycle.
The carbamoyl phosphate formation reaction is carried out in the mitochondrial matrix. Carbamoyl is the prefix used to represent an amide group. Thus carbamoyl phosphate is a molecule that contains a phosphate group attached to an amide functional group.
Depending upon the number of nitrogen atom present in the structure of the compound it can be classified as
(a)
Answer to Problem 26.75EP
Carbamoyl phosphate is a
Explanation of Solution
Carbamoyl phosphate and aspartate are fuel for the urea cycle. The structure of carbamoyl phosphate is:
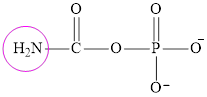
It contains only one nitrogen atom thus carbamoyl phosphate is characterized as
(b)
Interpretation: To classify glutamate in terms of nitrogen content as
Concept introduction: Glutamate is conjugate anion of glutamic acid. Glutamic acid is an amino acid. Depending upon the number of nitrogen atom present in the structure of the compound it can be classified as
(b)
Answer to Problem 26.75EP
Glutamate is a
Explanation of Solution
The structure of glutamate is:
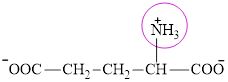
It contains only one nitrogen atom thus glutamate is characterized a
(c)
Interpretation: To characterize urea in terms of nitrogen content as
Concept introduction: The nitrogenous product of protein
Depending upon the number of nitrogen atom present in the structure of the compound it can be classified as
(c)
Answer to Problem 26.75EP
Urea is a
Explanation of Solution
The structure of urea is:
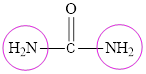
The structure of urea suggests the presence of two
(d)
Interpretation: To characterize citrulline in terms of nitrogen content as
Concept introduction: Citrulline is a nonstandard amino acid and is an inetrmediate in the the urea cycle. It is encountered at step 2 of the urea cycle and undergo condensation reaction with aspartate to give argininosuccinate.
Depending upon the number of nitrogen atom present in the structure of the compound it can be classified as
(d)
Answer to Problem 26.75EP
Citrulline is a
Explanation of Solution
The structure of citrulline is:
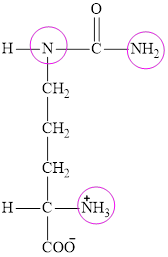
Citrulline contains three nitrogen atoms and thus it is characterized as a
Want to see more full solutions like this?
Chapter 26 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forward
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





