
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25.8, Problem 20P
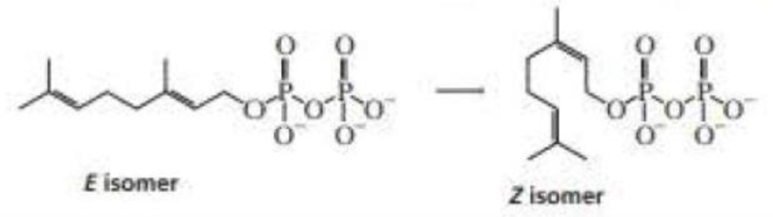
Propose a mechanism for the conversion of the E isomer of geranyl pyrophosphate to the Z isomer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If we assume a system with an anodic overpotential, the variation of n as a function
of current density:
1. at low fields is linear 2. at higher fields, it follows Tafel's law
Obtain the range of current densities for which the overpotential has the same value
when calculated for 1 and 2 cases (maximum relative difference of 5% compared to
the behavior for higher fields).
To which overpotential range does this correspond?
Data: i = 1.5 mA cm², T = 300°C, B = 0.64, R = 8.314 J K1 mol-1 and F = 96485 C mol-1.
Answer by equation please
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
Chapter 25 Solutions
Organic Chemistry (8th Edition)
Ch. 25.1 - Prob. 1PCh. 25.3 - Which has a higher melting point, glyceryl...Ch. 25.3 - Draw the structure of an optically inactive fat...Ch. 25.3 - Draw the structure of an optically active fat...Ch. 25.5 - Do the identities of R1 and R2 in phosphatidic...Ch. 25.5 - Membranes contain proteins, Integral membrane...Ch. 25.5 - Prob. 8PCh. 25.5 - The membrane phospholipids in deer have a higher...Ch. 25.6 - Treating PGC2 with a strong base such as sodium...Ch. 25.7 - Mark off the isoprene units in menthol, -selinene,...
Ch. 25.7 - Prob. 13PCh. 25.7 - Prob. 14PCh. 25.8 - Propose mechanisms for the Claisen condensation...Ch. 25.8 - Prob. 16PCh. 25.8 - Propose a mechanism for the conversion of...Ch. 25.8 - Propose a mechanism for the biosynthesis of...Ch. 25.8 - Propose a mechanism for the conversion of the E...Ch. 25.8 - The fluoro-substitued geranyl pyrophosphate shown...Ch. 25.8 - Prob. 22PCh. 25.8 - Prob. 23PCh. 25.9 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 25.10 - Prob. 26PCh. 25.10 - Prob. 27PCh. 25.10 - The acid component of a cholesterol ester is a...Ch. 25.10 - Prob. 29PCh. 25.10 - Prob. 30PCh. 25 - Prob. 31PCh. 25 - An optically active fat, when completely...Ch. 25 - Prob. 33PCh. 25 - a. How many different triacylglycerols are there...Ch. 25 - Cardiolipins are found in heart muscles. Draw the...Ch. 25 - Nutmeg contains a simple, fully saturated...Ch. 25 - Draw the product that is obtained from the...Ch. 25 - Prob. 39PCh. 25 - Prob. 40PCh. 25 - Propose a mechanism for the biosynthesis of...Ch. 25 - 5-Androstene-3.17-dione is isomerized to...Ch. 25 - Prob. 44PCh. 25 - Eudesmol is a sesquiterpene found in eucalyptus....Ch. 25 - Prob. 46PCh. 25 - Prob. 47PCh. 25 - Diethylstilbestrol (DES) was given to pregnant...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?arrow_forwardUsing the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forward
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you


 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License