
Concept explainers
(a)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate butyrate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of
(a)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid butyrate intermediate is succinate.
Explanation of Solution
Intermediates involved in the lipogenesis are derivative of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
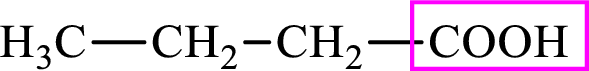
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a
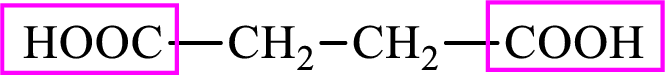
The structure of butyrate is,
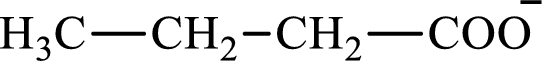
The structure of succinate is,
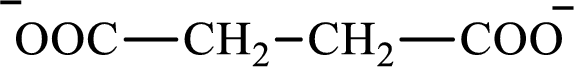
Butyrate and succinate are saturated acids with four carbon atoms in each molecule. Butyrate is a monoacid that is formed as an intermediate in lipogenesis while succinate is a diacid that is formed as an intermediate in the citric acid cycle. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid butyrate intermediate is succinate.
(b)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate acetoacetate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
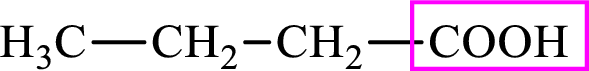
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
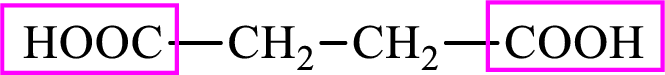
(b)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid acetoacetate intermediate is oxaloacetate.
Explanation of Solution
Acetoacetate is the intermediate in the lipogenesis. The structure of acetoacetate is,
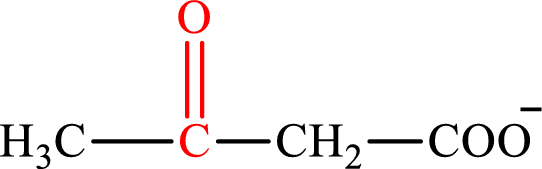
Oxaloacetate is the intermediate in the citric acid cycle. The structure of oxaloacetate is,
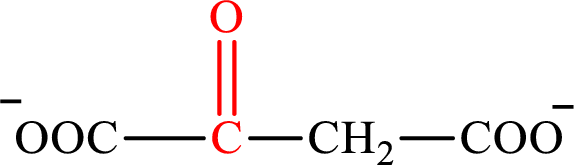
Acetoacetate and oxaloacetate are the keto derivatives of saturated acid with four carbon atoms in each molecule. Acetoacetate is a keto derivative of monocarboxylic acid while oxaloacetate is a keto derivative of dicarboxylic acid. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid acetoacetate intermediate is oxaloacetate.
(c)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate β-hydroxybutyrate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
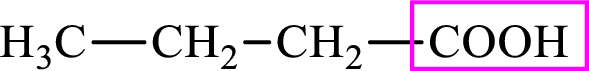
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
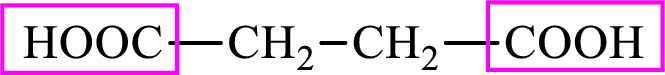
(c)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid β-hydroxybutyrate intermediate is malate.
Explanation of Solution
β-Hydroxybutyrate is the intermediate in the lipogenesis. The structure of β-hydroxybutyrate is,
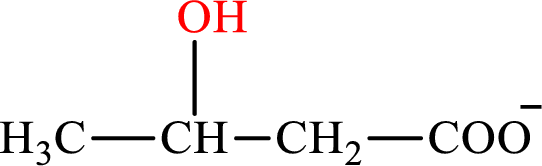
Malate is the intermediate in the citric acid cycle. The structure of Malate is,
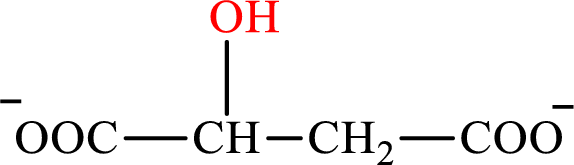
β-Hydroxybutyrate and malate are the hydroxy derivatives of saturated acid with four carbon atoms in each molecule. β-Hydroxybutyrate is a hydroxy derivative of monocarboxylic acid while malate is a hydroxy derivative of dicarboxylic acid. Therefore, the citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid β-hydroxybutyrate intermediate is malate.
(d)
Interpretation:
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid intermediate crotonate has to be determined.
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. This process is the reverse of the degradation of fatty acid.
The Citric acid cycle is a series of biochemical reactions that use acetyl CoA (produced by oxidation of pyruvate) to produce carbon dioxide, NADH and FADH2 in a series of redox reactions.
Intermediates involved in the lipogenesis are derivatives of C4 molecule butyric acid. Butyric acid is a monocarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the lipogenesis is a C4 derivative of monocarboxylic acid. The structure of butyric acid is,
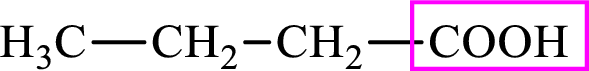
Succinic acid is a dicarboxylic acid and has 4 carbon atoms. Thus, each intermediate of the citric acid cycle is a C4 derivative of dicarboxylic acid. The structure of succinic acid is,
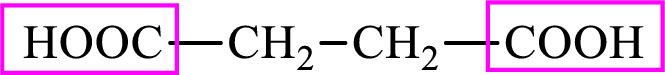
(d)
Answer to Problem 25.105EP
The citric acid cycle diacid intermediate counterpart for lipogenesis C4-ACP monoacid crotonate intermediate is fumarate.
Explanation of Solution
Crotonate is the intermediate in the lipogenesis. The structure of crotonate is,
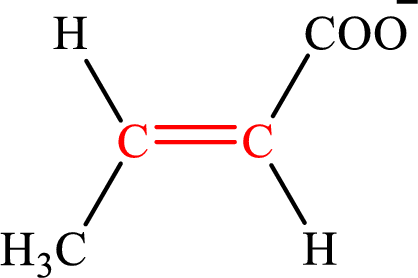
Fumarate is the intermediate in the citric acid cycle. The structure of fumarate is,
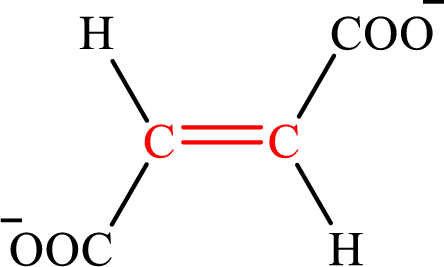
Crotonate and fumarate are the unsaturated derivatives of a
Want to see more full solutions like this?
Chapter 25 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning




