
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
7th Edition
ISBN: 8220100853180
Author: STOKER
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24.3, Problem 3QQ
Interpretation Introduction
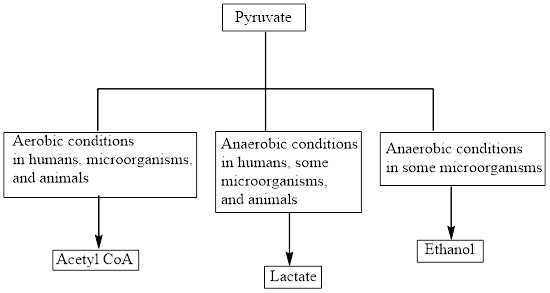
Interpretation: To identify the product of lactate fermentation.
Concept introduction: Pyruvate is the end product in the glycolysis. The glycolysis pathway takes place under anaerobic conditions. Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen.
The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The three common fates of pyruvate are as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.
Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.
Chapter 24 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
Ch. 24.1 - The primary site within the human body where...Ch. 24.1 - What is the first site within the human body where...Ch. 24.1 - What effect does gastric juice in the stomach have...Ch. 24.1 - Prob. 4QQCh. 24.1 - Which of the following substances is needed for...Ch. 24.2 - Prob. 1QQCh. 24.2 - Prob. 2QQCh. 24.2 - Prob. 3QQCh. 24.2 - Prob. 4QQCh. 24.2 - Prob. 5QQ
Ch. 24.2 - Prob. 6QQCh. 24.2 - Prob. 7QQCh. 24.3 - Prob. 1QQCh. 24.3 - Prob. 2QQCh. 24.3 - Prob. 3QQCh. 24.3 - Prob. 4QQCh. 24.3 - Accumulation of which of the following substances...Ch. 24.4 - Prob. 1QQCh. 24.4 - The net yield of ATP for the complete oxidation of...Ch. 24.4 - Prob. 3QQCh. 24.5 - Prob. 1QQCh. 24.5 - Prob. 2QQCh. 24.5 - Prob. 3QQCh. 24.6 - Prob. 1QQCh. 24.6 - Prob. 2QQCh. 24.6 - Prob. 3QQCh. 24.6 - Which of the following statements about ATP...Ch. 24.6 - Prob. 5QQCh. 24.7 - Prob. 1QQCh. 24.7 - Prob. 2QQCh. 24.8 - Prob. 1QQCh. 24.8 - Prob. 2QQCh. 24.8 - Prob. 3QQCh. 24.9 - Which of the following hormones promotes the...Ch. 24.9 - Which of the following pairs of hormones increases...Ch. 24.10 - Prob. 1QQCh. 24.10 - Prob. 2QQCh. 24.10 - Prob. 3QQCh. 24 - Where does carbohydrate digestion begin in the...Ch. 24 - Very little digestion of carbohydrates occurs in...Ch. 24 - Prob. 24.3EPCh. 24 - Prob. 24.4EPCh. 24 - Prob. 24.5EPCh. 24 - Prob. 24.6EPCh. 24 - Prob. 24.7EPCh. 24 - Prob. 24.8EPCh. 24 - Prob. 24.9EPCh. 24 - Prob. 24.10EPCh. 24 - Prob. 24.11EPCh. 24 - Prob. 24.12EPCh. 24 - Prob. 24.13EPCh. 24 - Prob. 24.14EPCh. 24 - Prob. 24.15EPCh. 24 - Prob. 24.16EPCh. 24 - Prob. 24.17EPCh. 24 - Prob. 24.18EPCh. 24 - Prob. 24.19EPCh. 24 - Prob. 24.20EPCh. 24 - Prob. 24.21EPCh. 24 - Prob. 24.22EPCh. 24 - Prob. 24.23EPCh. 24 - Prob. 24.24EPCh. 24 - Prob. 24.25EPCh. 24 - Prob. 24.26EPCh. 24 - Prob. 24.27EPCh. 24 - Prob. 24.28EPCh. 24 - Prob. 24.29EPCh. 24 - Prob. 24.30EPCh. 24 - Prob. 24.31EPCh. 24 - Prob. 24.32EPCh. 24 - Prob. 24.33EPCh. 24 - Prob. 24.34EPCh. 24 - Prob. 24.35EPCh. 24 - Prob. 24.36EPCh. 24 - Prob. 24.37EPCh. 24 - Prob. 24.38EPCh. 24 - Prob. 24.39EPCh. 24 - Prob. 24.40EPCh. 24 - Prob. 24.41EPCh. 24 - Prob. 24.42EPCh. 24 - Prob. 24.43EPCh. 24 - Prob. 24.44EPCh. 24 - Prob. 24.45EPCh. 24 - Prob. 24.46EPCh. 24 - Prob. 24.47EPCh. 24 - Prob. 24.48EPCh. 24 - Prob. 24.49EPCh. 24 - Prob. 24.50EPCh. 24 - Prob. 24.51EPCh. 24 - Prob. 24.52EPCh. 24 - Prob. 24.53EPCh. 24 - Prob. 24.54EPCh. 24 - Prob. 24.55EPCh. 24 - Prob. 24.56EPCh. 24 - Prob. 24.57EPCh. 24 - Prob. 24.58EPCh. 24 - Prob. 24.59EPCh. 24 - Prob. 24.60EPCh. 24 - Prob. 24.61EPCh. 24 - Prob. 24.62EPCh. 24 - Prob. 24.63EPCh. 24 - Prob. 24.64EPCh. 24 - Prob. 24.65EPCh. 24 - The liver, but not the brain or muscle cells, has...Ch. 24 - Prob. 24.67EPCh. 24 - Prob. 24.68EPCh. 24 - Prob. 24.69EPCh. 24 - Prob. 24.70EPCh. 24 - Prob. 24.71EPCh. 24 - Prob. 24.72EPCh. 24 - Prob. 24.73EPCh. 24 - Prob. 24.74EPCh. 24 - Prob. 24.75EPCh. 24 - Prob. 24.76EPCh. 24 - Prob. 24.77EPCh. 24 - Prob. 24.78EPCh. 24 - Prob. 24.79EPCh. 24 - Prob. 24.80EPCh. 24 - Prob. 24.81EPCh. 24 - Prob. 24.82EPCh. 24 - Prob. 24.83EPCh. 24 - Prob. 24.84EPCh. 24 - Prob. 24.85EPCh. 24 - Prob. 24.86EPCh. 24 - Prob. 24.87EPCh. 24 - Prob. 24.88EPCh. 24 - Prob. 24.89EPCh. 24 - Prob. 24.90EPCh. 24 - Prob. 24.91EPCh. 24 - Prob. 24.92EPCh. 24 - Prob. 24.93EPCh. 24 - Prob. 24.94EPCh. 24 - Prob. 24.95EPCh. 24 - Prob. 24.96EPCh. 24 - Prob. 24.97EPCh. 24 - Prob. 24.98EPCh. 24 - Prob. 24.99EPCh. 24 - Prob. 24.100EPCh. 24 - Prob. 24.101EPCh. 24 - Prob. 24.102EPCh. 24 - Prob. 24.103EPCh. 24 - Prob. 24.104EPCh. 24 - Prob. 24.105EPCh. 24 - Prob. 24.106EPCh. 24 - Prob. 24.107EPCh. 24 - Prob. 24.108EPCh. 24 - Prob. 24.109EPCh. 24 - Prob. 24.110EPCh. 24 - Prob. 24.111EPCh. 24 - Prob. 24.112EPCh. 24 - Prob. 24.113EPCh. 24 - Prob. 24.114EPCh. 24 - Prob. 24.115EPCh. 24 - Compare the biological functions of glucagon and...Ch. 24 - Prob. 24.117EPCh. 24 - Prob. 24.118EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
- Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning