
Concept explainers
(a)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:
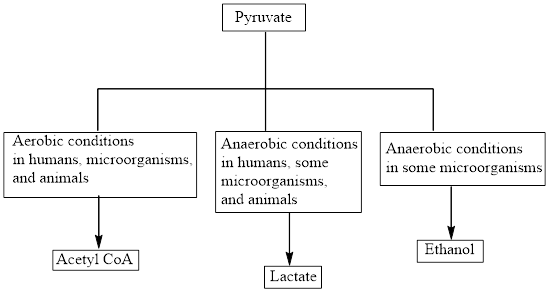
Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
(a)
Answer to Problem 24.45EP
Carbon dioxide
Explanation of Solution
Reason for correct choice:
Under aerobic conditions, pyruvate is converted to
The process of ethanol fermentation takes place in two steps. In step 1, the pyruvate molecule is converted to acetaldehyde by pyruvate decarboxylase enzymes. Carbon dioxide molecule is produced in this step. In step 2, acetaldehyde is reduced to ethanol by alcohol dehydrogenase enzymes. The ethanol fermentation equation is as follows:
Therefore,
Reason for incorrect choice:
The reaction equation for lactate fermentation is as follows:
(b)
Interpretation: To identify NADH is a reactant in which the fate of pyruvate-
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions.
The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the
A reactant is defined as the substance that is initially present in the
(b)
Answer to Problem 24.45EP
NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore, NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to
Therefore, NADH is formed along with
(c)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the redox reactions in metabolism. Its reduced form is NADH and oxidized form is
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
(c)
Answer to Problem 24.45EP
In the production of
Explanation of Solution
Reason for correct choice:
The reaction equation for the conversion of pyruvate to
Therefore,
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to lactate is as follows:
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore,
(d)
Interpretation: To identify the end product is a
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Pyruvate
(d)
Answer to Problem 24.45EP
In the absence of oxygen, pyruvate is converted to
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. This anaerobic reduction is called lactate fermentation. The chemical reaction for the formation of lactate is as follows:

Lactate contains three carbon atoms. Therefore, lactate is a
Reason for incorrect choice:
In the ethanol fermentation process, pyruvate is converted to ethanol and carbon dioxide by enzymes under the anaerobic conditions. The ethanol fermentation equation is as follows:
Ethanol
Pyruvate is converted to
Acetyl group
Want to see more full solutions like this?
Chapter 24 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




