
(a)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).
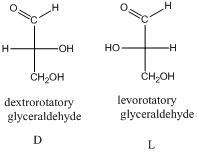
The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (a) is D or L sugar and the configuration of each chiral center.
(b)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (b) is D or L sugar and the configuration of each chiral center.
(c)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (c) is D or L sugar and the configuration of each chiral center.
(d)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (d) is D or L sugar and the configuration of each chiral center.
(e)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To identify: the given carbohydrate (e) is D or L sugar and the configuration of each chiral center.
(f)
Interpretation:
For each of the given compounds, whether it is D or L sugar should be determined, the configuration of each chiral center should be assigned and the trend on the configuration of each chiral center should be explained.
Concept introduction:
- The stereo-descriptor used for carbohydrates is D or L. it is based on the dextrorotatory or levorotatory of smallest carbohydrate glyceraldehyde (1 chiral center).

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Chiral carbon: Chiral carbon is the one which is bonded to four different molecules or groups.
- Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
- Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
- If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
- If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
- If the least priority group is on horizontal line in the fisher projection, then configuration is inverted to the obtained configuration from the above CIP rule which means R configuration becomes S and vice versa.
- The D-configuration is needed not to be dextrorotatory; rather it means the chirality center of farthest from aldo-group is having R-configuration or the –OH group is on right side.
To explain: the trend on the configuration of each chiral center in each given carbohydrates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry, Binder Ready Version
- The electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forwardHello, I am doing a court case analysis in my Analytical Chemistry course. The case is about a dog napping and my role is prosecution of the defendant. I am tasked in the Area of Expertise in Neutron Activation and Isotopic Analysis. Attached is the following case study reading of my area of expertise! The landscaping stone was not particularly distinctive in its decoration but matched both the color and pattern of the Fluential’s landscaping stone as well as the stone in the back of the recovered vehicle. Further analysis of the stone was done using a technique called instrumental neutron activation analysis. (Proceed to Neutron Activation data) Photo Notes: Landscaping stone recovered in vehicle. Stone at Fluential’s home is similar inappearance. Finally, the white paint on the brick was analyzed using stable isotope analysis. The brick recovered at the scene had smeared white paint on it. A couple of pieces of brick in the back of the car had white paint on them. They…arrow_forwardCite the stability criteria of an enamine..arrow_forward
- What would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forwardWhat is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forward
- Please complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forwardWhat would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





