
(a)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
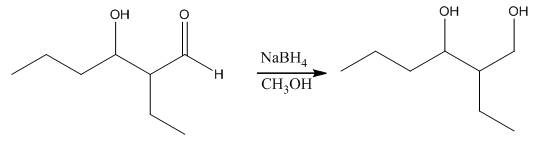
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown as,
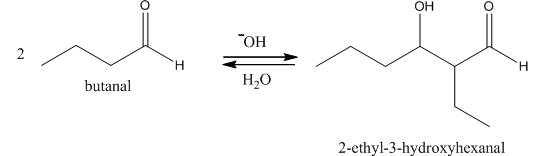
Figure 1
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert

Figure 2
Thus, the reagent that is needed to convert
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(b)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
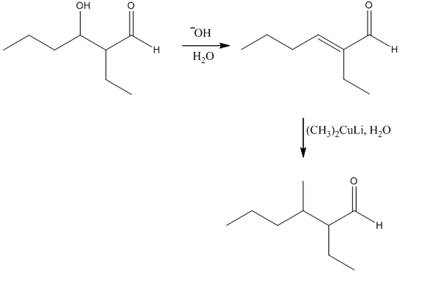
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert
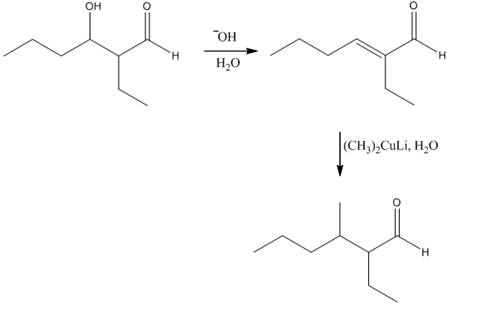
Figure 3
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(c)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
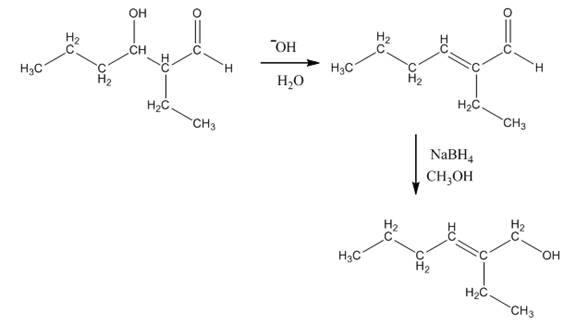
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert
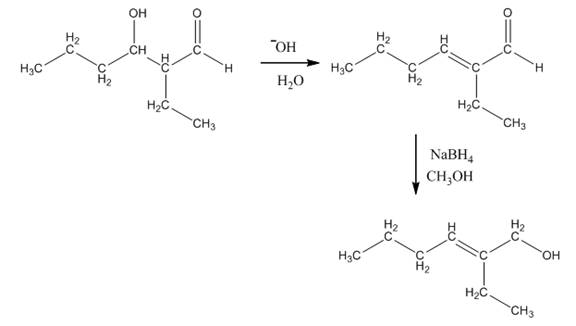
Figure 4
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
(d)
Interpretation: The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is to be shown. The reagents that are needed to convert the obtained product to the given compound are to be predicted.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.9P
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
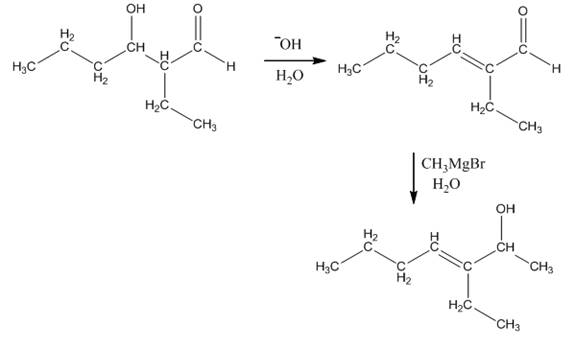
Explanation of Solution
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is shown in Figure 1.
In this reaction, first of all, one equivalent of butanal is treated with the strong base that results in the formation of a resonance-stabilized enolate ion. This enolate ion reacts with the second equivalent of butanal followed by the hydrolysis that leads to the formation of the desired product,
The reagents that are needed to convert

Figure 5
In this reaction,
The aldol product that is formed by the reaction of the two molecules of butanal in the presence of base is
Want to see more full solutions like this?
Chapter 24 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


