
Concept explainers
Answer the following questions about
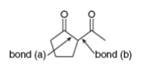
a. What starting materials are needed to form
b. What starting materials are needed to form
c. What product is f ormed when
d. Draw the Robinson annulation product(s) formed by reaction of
e. Draw the structure of the most stable enol tautomer(s).
(a)
Interpretation: The starting materials needed to form
Concept introduction: In crossed Claisen condensation reaction, a base abstracts an acidic proton from an
Answer to Problem 24.65P
The starting materials needed to form
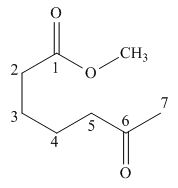
Figure 1
Explanation of Solution
The given compound is,
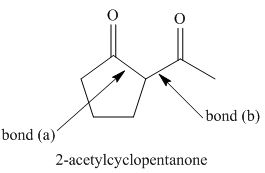
Figure 2
The starting materials needed to form

Figure 1
The base abstracts the proton from
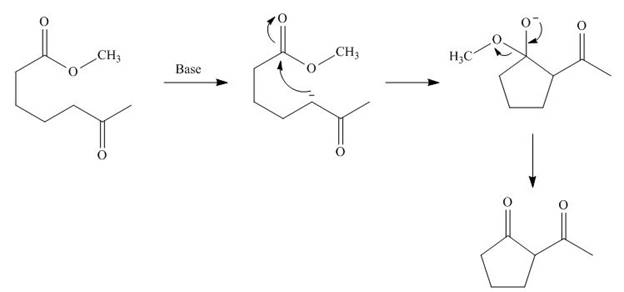
Figure 3
The starting materials needed to form
(b)
Interpretation: The starting materials needed to form
Concept introduction: In crossed Claisen condensation reaction, a base abstracts an acidic proton from an
Answer to Problem 24.65P
The starting materials needed to form
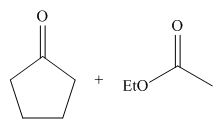
Figure 4
Explanation of Solution
The given compound is,

Figure 2
The starting materials needed to form

Figure 4
The base abstracts the proton from
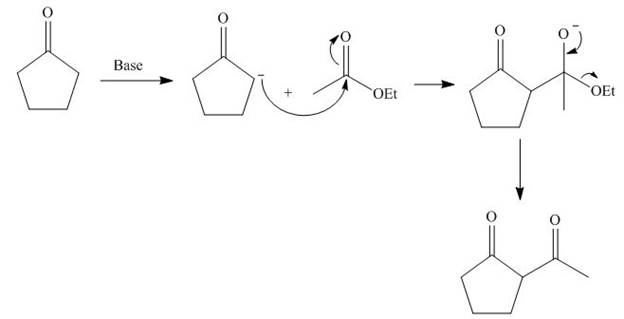
Figure 5
The starting materials needed to form
(c)
Interpretation: The product formed on treatment of
Concept introduction: Alkylation of carbonyl compounds takes place in the presence of a strong base. The use of appropriate base, solvent and temperature can yield one major product regioselectively. The first step is abstraction of proton and second step is attack of electrophile to form alkylated product.
Answer to Problem 24.65P
The product formed on treatment of
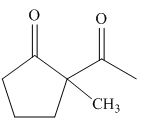
Figure 6
Explanation of Solution
The product formed on treatment of
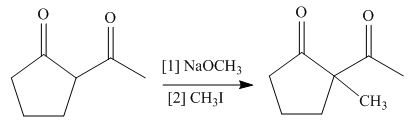
Figure 7
The base
The product formed on treatment of
(d)
Interpretation: The products formed on Robinson annulations of
Concept introduction: Robinson annulation is a combination of Michael addition and intramolecular aldol condensation reactions. It takes place between
Answer to Problem 24.65P
The products formed on Robinson annulations of
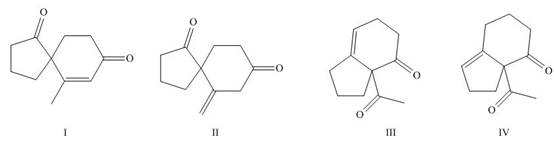
Figure 8
Explanation of Solution
The first step is the Michael addition between
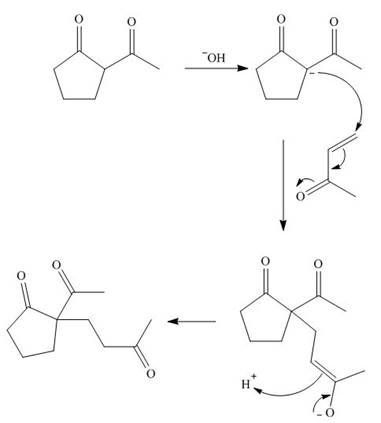
Figure 9
The base abstracts the proton between the two carbonyl groups to form enolate. The enolate formed gives the conjugate addition on methyl vinyl ketone followed by protonation resulting in
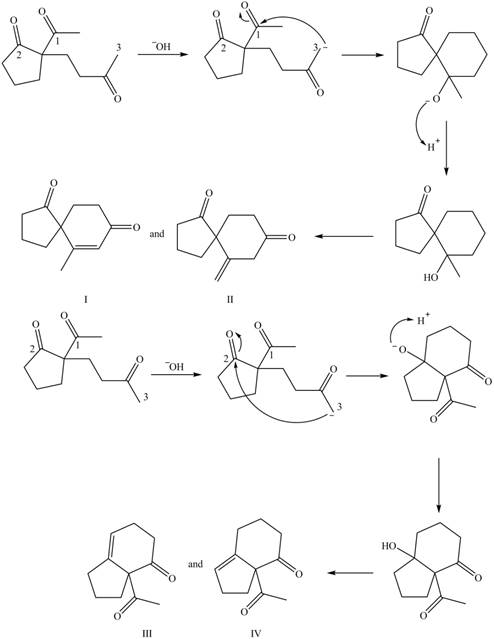
Figure 10
The base abstracts the acidic proton from the
The products formed on Robinson annulations of
(e)
Interpretation: The structure of the most stable enol form tautomer is to be drawn.
Concept introduction: Keto and enol form of a compound exists in the chemical equilibrium with each other. Enol form refers to the compound in which carbonyl group exist as hydroxyl group adjacent to carbon-carbon double bond.
Answer to Problem 24.65P
The structure of the most stable enol form tautomer is,
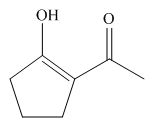
Figure 11
Explanation of Solution
The enol form tautomer of
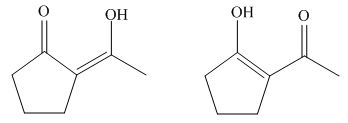
Figure 12
The stability of enols depends on the stability of alkenes. The alkene with endo double bond is more stable in case of five membered ring.
Thus, the most stable enol form tautomer is,

Figure 11
The structure of the most stable enol form tautomer is shown in Figure 11.
Want to see more full solutions like this?
Chapter 24 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
