
Concept explainers
(a)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
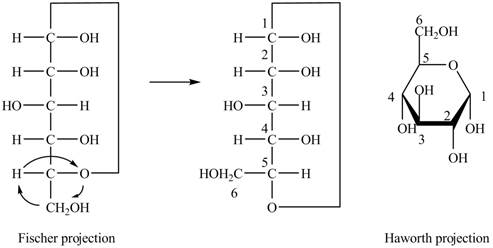
The Fischer projection of
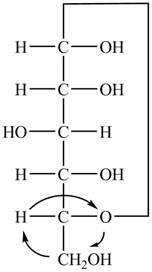
Figure 1
The Fischer projection of
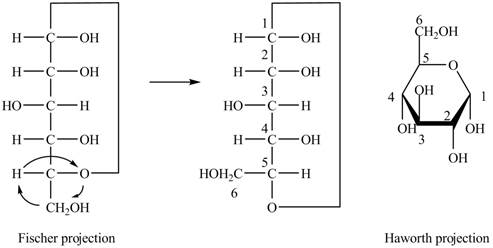
Figure 2
The chair conformation of
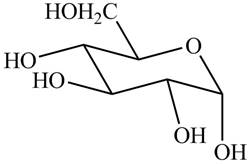
Figure 3
The Fischer projection, Haworth projection, and a line-and wedge structure for
(b)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
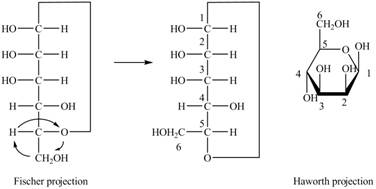
Explanation of Solution
The Fischer projection of
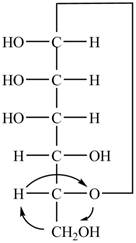
Figure 4
The Fischer projection of

Figure 5
The chair conformation of
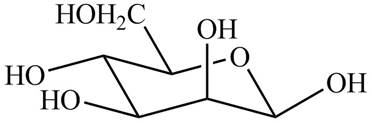
Figure 6
The Fischer projection, Haworth projection, and a line-and wedge structure for
(c)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
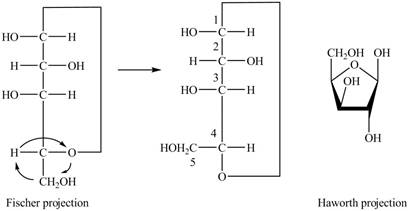
Explanation of Solution
The Fischer projection of
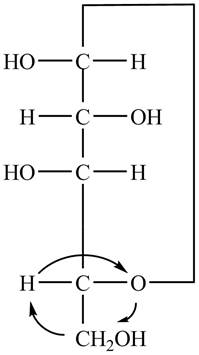
Figure 7
The Fischer projection of
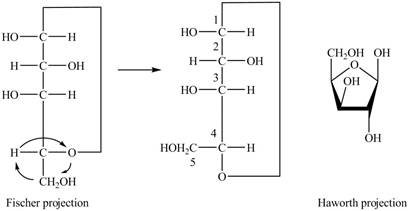
Figure 8
The chair conformation of
The Fischer projection, Haworth projection, and a line-and wedge structure for
(d)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
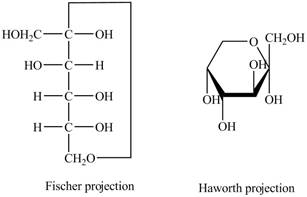
The Fischer projection of
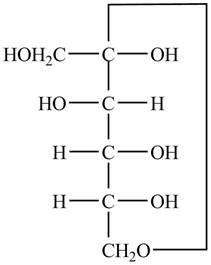
Figure 9
The Fischer projection of

Figure 10
The chair conformation of
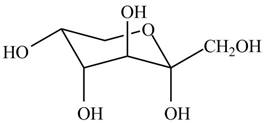
Figure 11
The Fischer projection, Haworth projection, and a line-and wedge structure for
(e)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
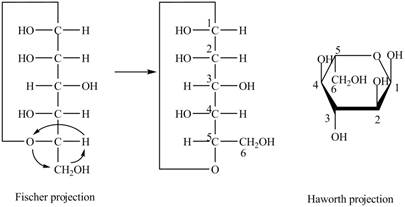
Explanation of Solution
The Fischer projection of
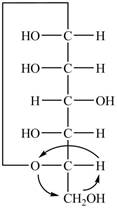
Figure 12
The Fischer projection of

Figure 13
The chair conformation of
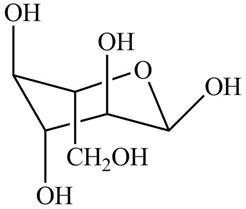
Figure 14
The Fischer projection, Haworth projection, and a line-and wedge structure for
(f)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
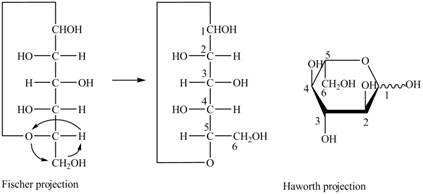
Explanation of Solution
The Fischer projection for a mixture of the
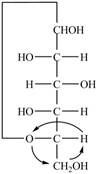
Figure 15
The Fischer projection for a mixture of the

Figure 16
The chair conformation for a mixture of the
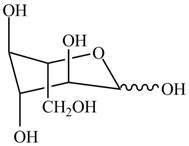
Figure 17
The Fischer projection, Haworth projection, and a line-and wedge structure for
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- The product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH₂CN H+ N Click and drag to start drawing a structure. X $arrow_forwardExplain what is the maximum absorbance of in which caffeine absorbs?arrow_forwardExplain reasons as to why the amount of caffeine extracted from both a singular extraction (5ml Mountain Dew) and a multiple extraction (2 x 5.0ml Mountain Dew) were severely high when compared to coca-cola?arrow_forward
- Protecting Groups and Carbonyls 6) The synthesis generates allethrolone that exhibits high insect toxicity but low mammalian toxicity. They are used in pet shampoo, human lice shampoo, and industrial sprays for insects and mosquitos. Propose detailed mechanistic steps to generate the allethrolone label the different types of reagents (Grignard, acid/base protonation, acid/base deprotonation, reduction, oxidation, witting, aldol condensation, Robinson annulation, etc.) III + VI HS HS H+ CH,CH,Li III I II IV CI + P(Ph)3 V ༼ Hint: no strong base added VI S VII IX HO VIII -MgBr HgCl2,HgO HO. isomerization aqeuous solution H,SO, ༽༽༤༽༽ X MeOH Hint: enhances selectivity for reaction at the S X ☑arrow_forwardDraw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forward
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

