
(a)
Interpretation:
Synthesis of 3-nitrophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of
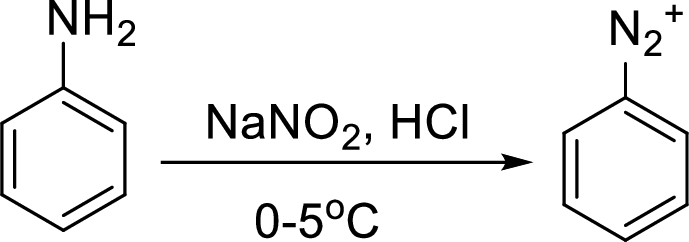
Aromatic amines converted to arenediazonium salt by reacting with
(b)
Interpretation:
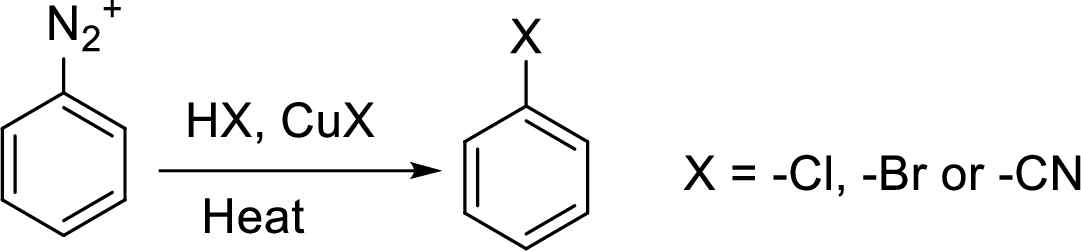
Synthesis of 3-bromo nitrobenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
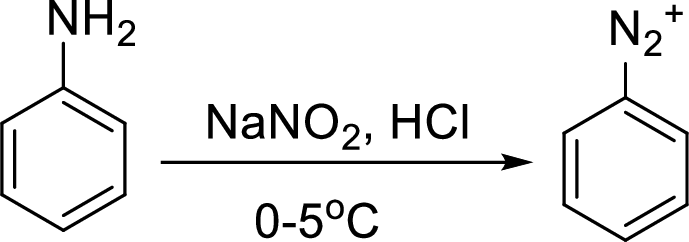
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
(c)
Interpretation:
Synthesis of 1,3-dihydroxybenzene has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
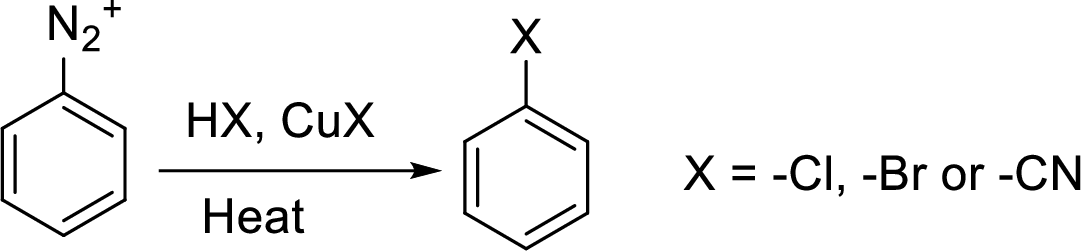
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
(d)
Interpretation:
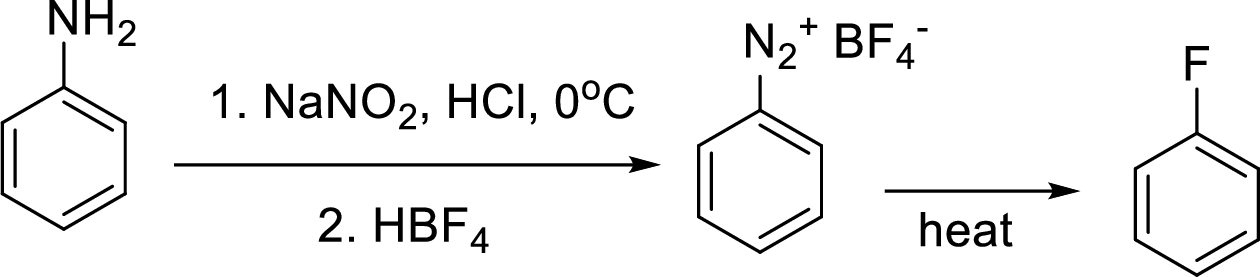
Synthesis of 3-fluoroaniline has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
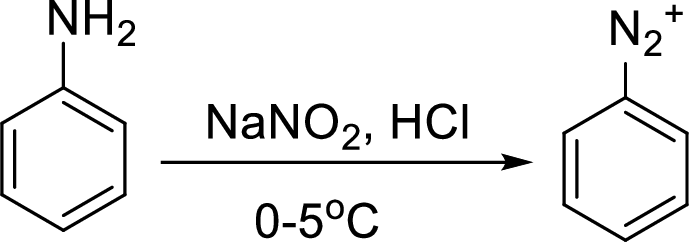
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
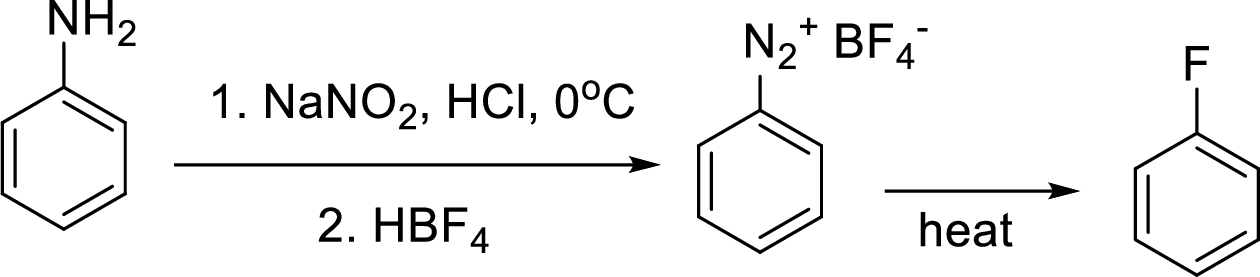
(e)
Interpretation:
Synthesis of 3-fluorophenol has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Conversion of aromatic amines to phenol:
Aromatic amines converted to arenediazonium salt by reacting with
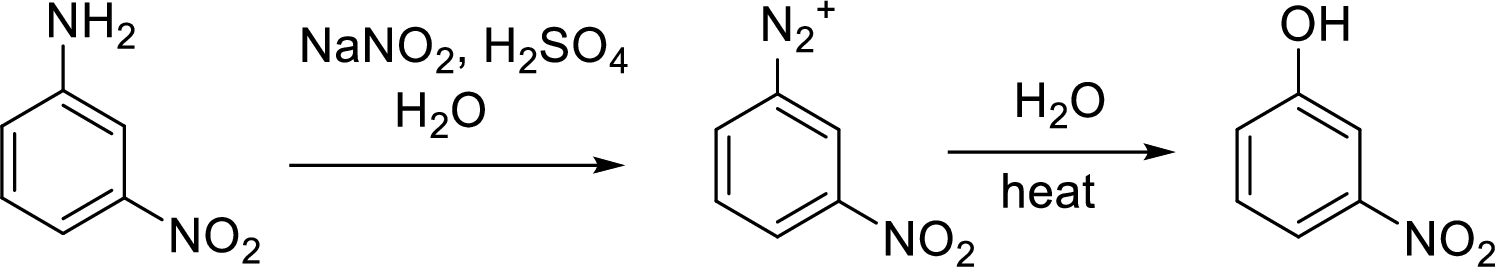
Schiemann reaction: It is a method used to introduce fluorine into an aromatic ring. The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
(f)
Interpretation:
Synthesis of 3-hydroxybenzonitrile has to be proposed using 3-nitroaniline as starting material.
Concept Introduction:
Hydrogenation:
Hydrogenation means the addition of hydrogen molecules to the unsaturated compound which makes them saturated hydrocarbon in the presence of catalyst.
Reaction of a primary aromatic amine with sodium nitrite:
The reaction of a primary aromatic amine with sodium nitrite in presence of aqueous
Sandmeyer reaction: It reaction type of organic reaction where the diazonium group in an arenediazonium salt gets replaced by
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
