
Concept explainers
(a)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or
Concept introduction:
In chemistry Structure is the arrangement of
Depending on the number of carbon side chain of the amide, different types of amides can form.
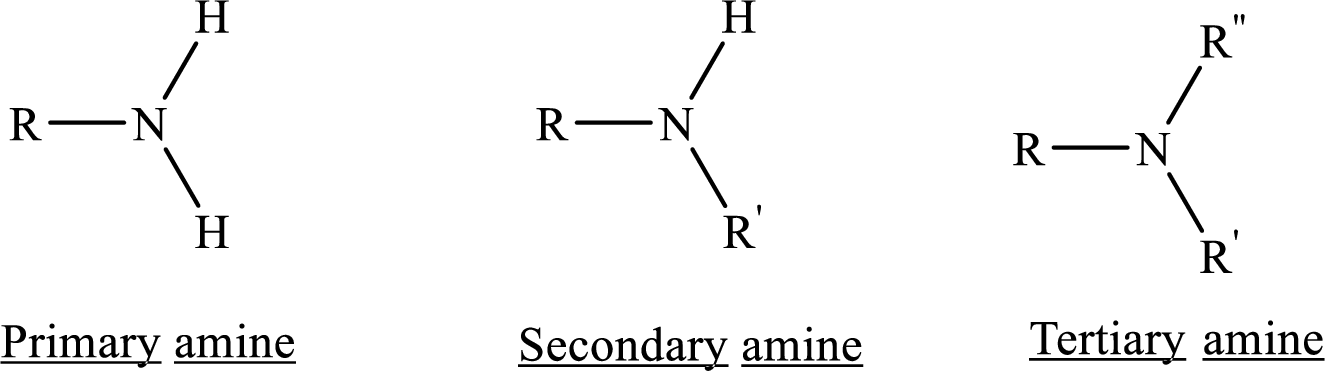
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine.
(b)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or aromatic.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
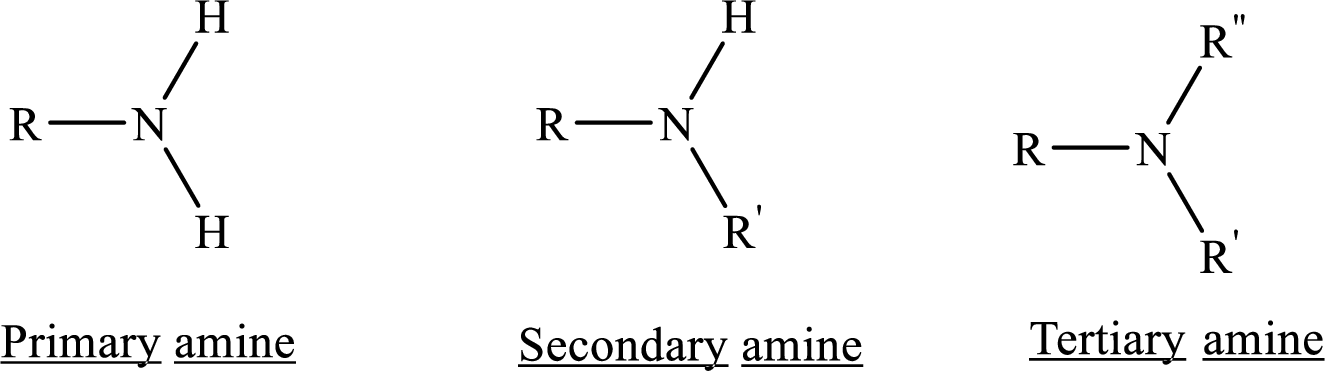
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine
(c)
Interpretation:
Each of the amine has to be classified as primary, secondary, or tertiary amine and as aliphatic or aromatic.
Concept introduction:
In chemistry Structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the amide, different types of amides can form.
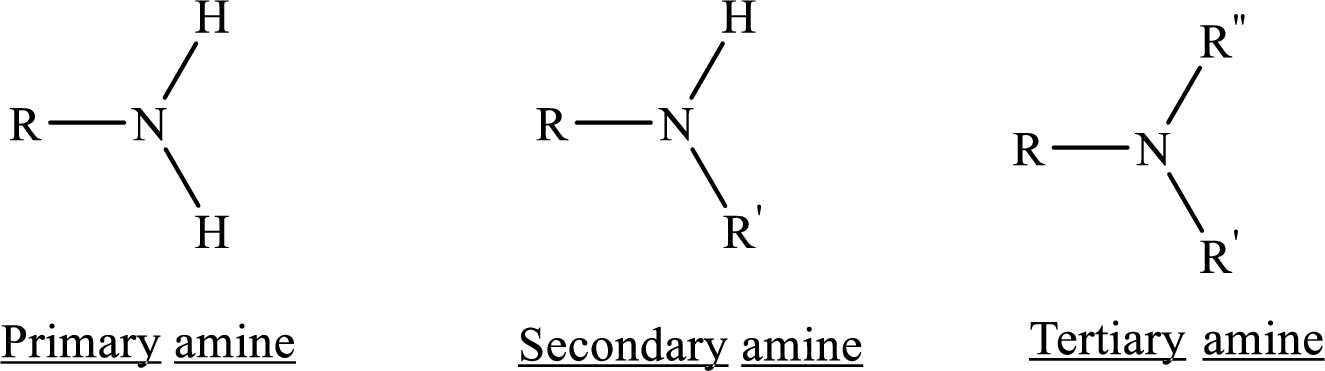
If nitrogen atom in amine group is attached only to alkyl groups, then it is an aliphatic amine.
If nitrogen atom in amine group is attached to at least one aryl groups, then it is an aromatic amine.
If nitrogen atom in amine group is a part of an aromatic ring, then it is a heterocyclic aromatic amine.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry, Loose-leaf Version
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
- Draw the monomers required to synthesize this condensation polymer.arrow_forward8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forward
- i need help on how to complete the followingarrow_forwardno AI walkthrough current image is wrong answerarrow_forwarda. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





