
Concept explainers
(a)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
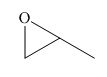
Figure 1
The nucleophile
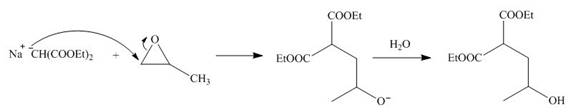
Figure 2
On the further treatment of
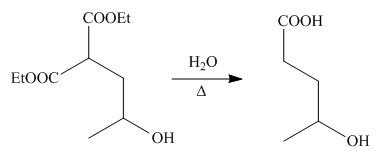
Figure 3
Hence, the product formed by the reaction of
The product formed by the reaction of
(b)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
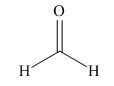
Figure 4
The nucleophile
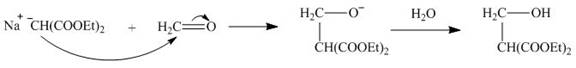
Figure 5
On the further treatment of
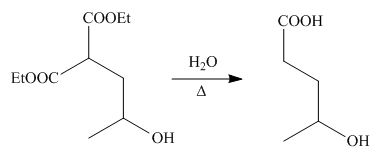
Figure 6
Hence, the product formed by the reaction of
The product formed by the reaction of
(c)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The product formed by the reaction of
Explanation of Solution
The given compound is,
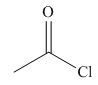
Figure 7
The nucleophile
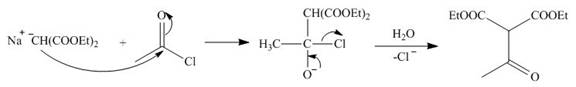
Figure 8
On the further treatment of
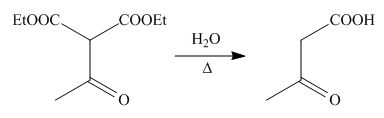
Figure 9
Hence, the product formed by the reaction of
The product formed by the reaction of
(d)
Interpretation: The product formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.49P
The products formed by the reaction of
Explanation of Solution
The given compound is,
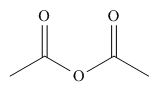
Figure 10
The nucleophile
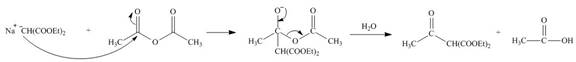
Figure 11
On the further treatment of
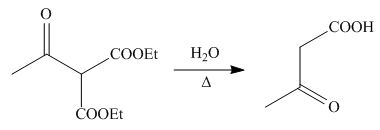
Figure 12
Hence, the products formed by the reaction of
The products formed by the reaction of
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- The reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward
- 20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forwardplease provide the structure for this problem, thank you!arrow_forwardDraw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forward
- please provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forward
- presented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

