
Concept explainers
(a)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.16P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
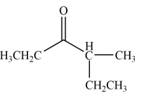
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
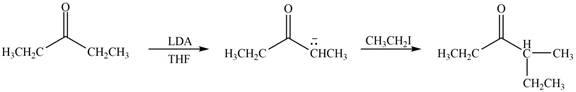
Figure 1
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom of the compound. Then methyl iodide reacts with the enolte ion to form the desired product.
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(b)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.16P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
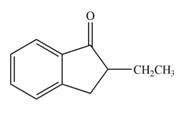
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
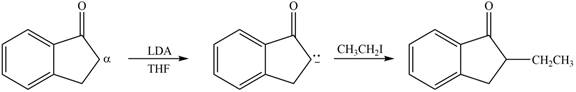
Figure 2
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(c)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.16P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
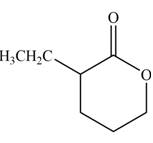
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
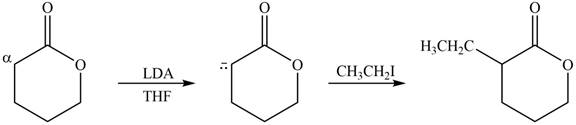
Figure 3
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(d)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.16P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
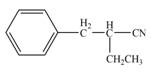
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
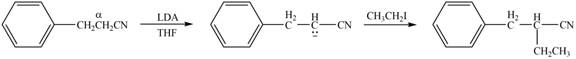
Figure 4
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





