
Concept explainers
(a)
Interpretation:
The iodo (–I) and formyl (–CHO) groups are not listed in Table 23-1. The relative amounts of ortho and meta nitration products obtained for each substituent are shown here:
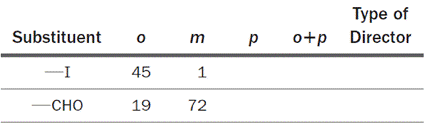
The missing information pertaining to the relative amounts of the para isomers that are produced as well as the sum of the amounts of ortho and para products are to be supplied.
Concept introduction:
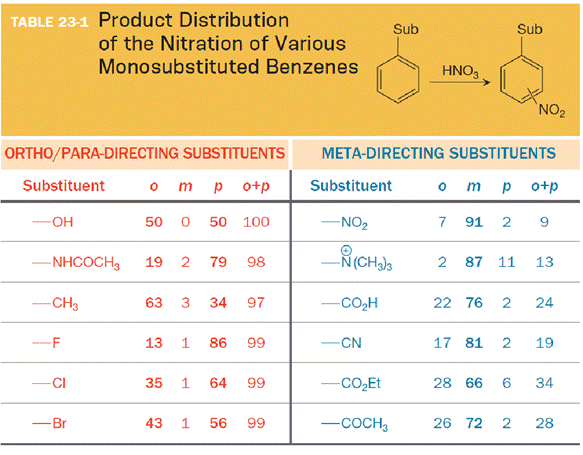
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
(b)
Interpretation:
The iodo (
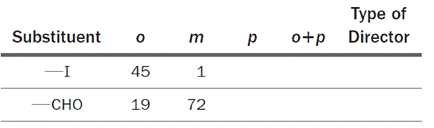
Based on the information, whether each substituent is an ortho/para director or a meta director is to be determined.
Concept introduction:
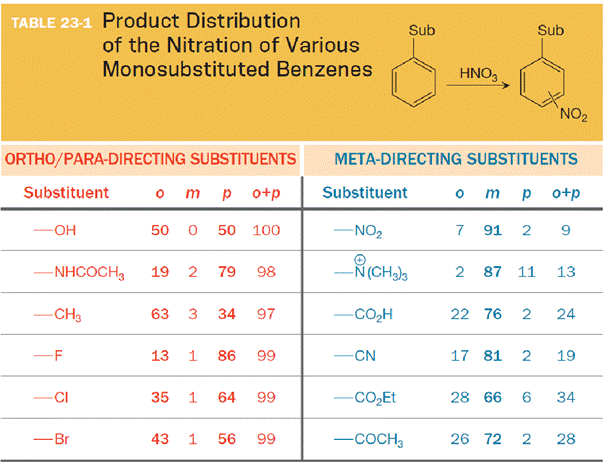
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Predict the products of this organic reaction: NaBH3CN + NH2 ? H+ Click and drag to start drawing a structure. ×arrow_forwardPredict the organic products that form in the reaction below: + OH +H H+ ➤ ☑ X - Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Garrow_forwardPredict the organic products that form in the reaction below: OH H+ H+ + ☑ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. ✓ marrow_forward
- Determine the structures of the missing organic molecules in the following reaction: + H₂O +H H+ Y Z ☑ ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw the structures in any arrangement that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure once. Click and drag to start drawing a structure. AP +arrow_forwardPlease help, this is all the calculations i got!!! I will rate!!!Approx mass of KMnO in vial: 3.464 4 Moss of beaker 3×~0. z Nax200: = 29.9219 Massof weacerv after remosimgain N2C2O4. Need to fill in all the missing blanks. ง ง Approx mass of KMnO4 in vials 3.464 Mass of beaker + 3x ~0-304: 29.9219 2~0.20 Miss of beaker + 2x- 29.7239 Mass of beaker + 1x~0.2g Naz (204 29-5249 Mass of beaver after removing as qa Na₂ C₂O T1 T2 T3 Final Buiet reading Initial butet reading (int)) Hass of NaOr used for Titration -reading (mL) calculation Results: 8.5ml 17mL 27.4mL Oml Om Oml T1 T2 T3 Moles of No CO Moles of KMO used LOF KM. O used Molenty of KMNO Averagem Of KMOWLarrow_forwardDraw the skeletal ("line") structure of 2-hydroxy-4-methylpentanal. Click and drag to start drawing a structure. Xarrow_forward
- Determine whether the following molecule is a hemiacetal, acetal, or neither and select the appropriate box below. Also, highlight the hemiacetal or acetal carbon if there is one. hemiacetal acetal Oneither OHarrow_forwardWhat is the missing reactant R in this organic reaction? ་ ་ ་ ་ ་ ་ ་ ་ ་ ་ +R H3O+ • Draw the structure of R in the drawing area below. N • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure.arrow_forwardWrite the systematic name of each organic molecule: H structure H OH OH H OH name ☐ OHarrow_forward
- Determine whether each of the following molecules is a hemiacetal, acetal, or neither and select the appropriate box in the table. CH3O OH OH OH hemiacetal acetal neither hemiacetal acetal neither Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N དལ་ད་་ + R • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. ㄖˋarrow_forwardDraw the condensed structure of 4-hydroxy-3-methylbutanal. Click anywhere to draw the first atom of your structure.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

