
Concept explainers
Interpretation:
The synthesis of each of the given compound from the indicated starting material is to be stated.
Concept introduction:
The hydration of
The reagent
Lithium aluminum hydride and sodium borohydride are strong reducing agents. They are inorganic compounds and are used as the reducing agents in organic synthesis. They are used for the conversion of carboxylic acids, aldehydes and ketones into primary and secondary alcohols.
Answer to Problem 49P
Solution:
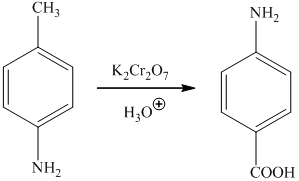
a) The synthesis of
b) The synthesis of
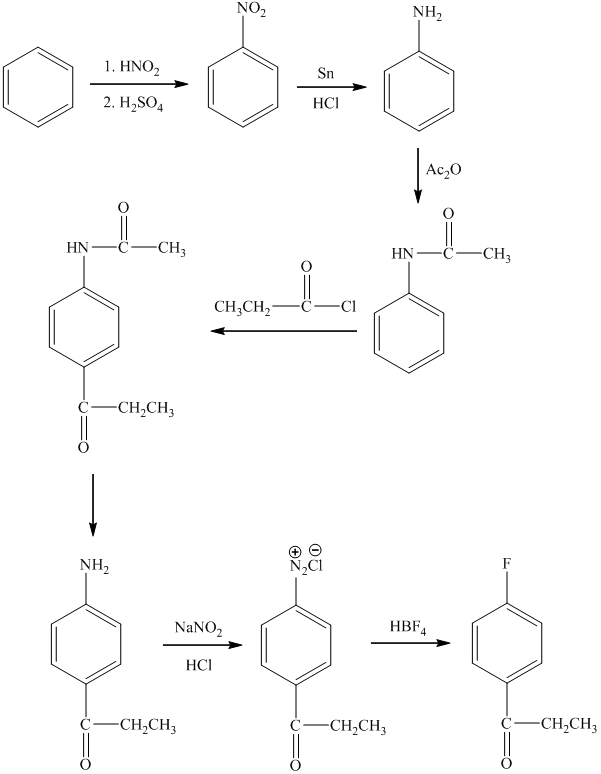
c) The synthesis of
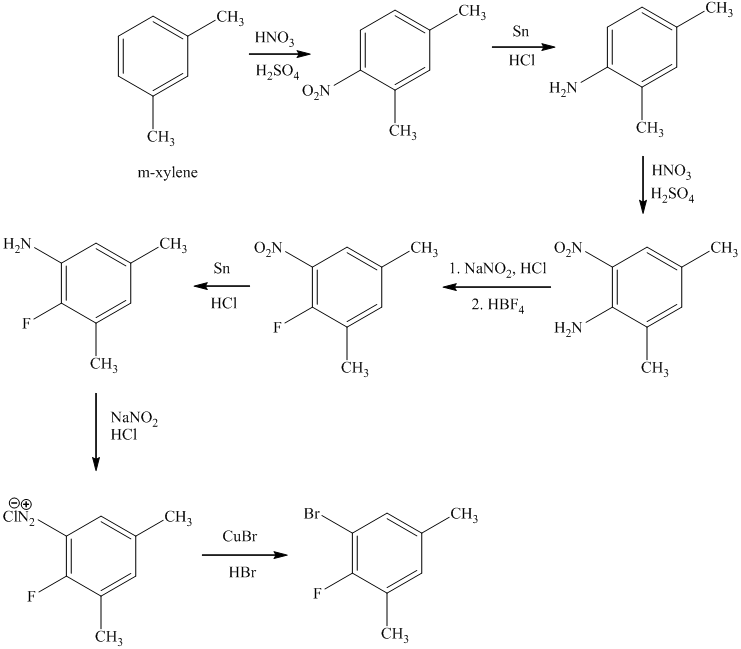
d) The synthesis of
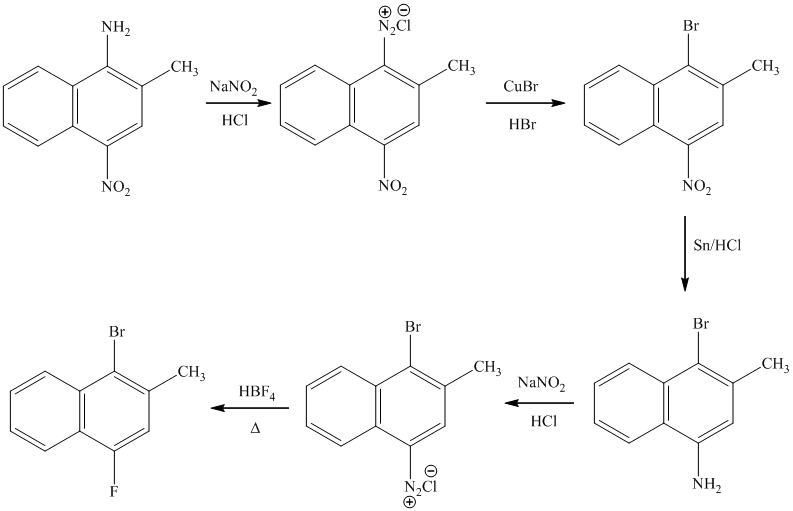
e) The synthesis of
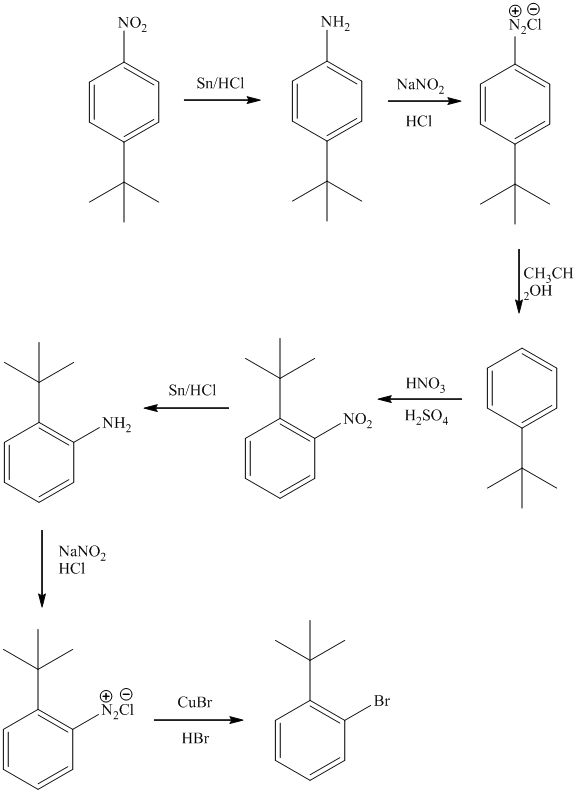
f) The synthesis of
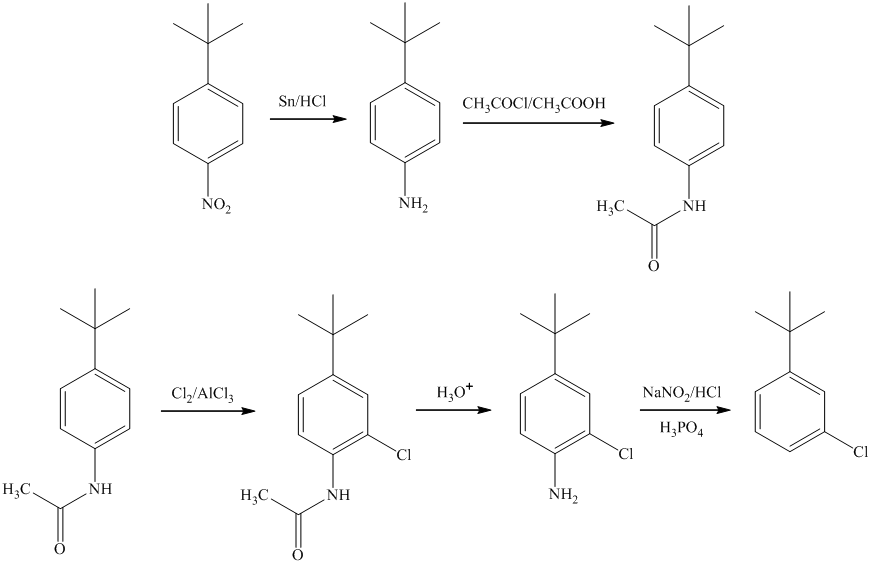
g) The synthesis of
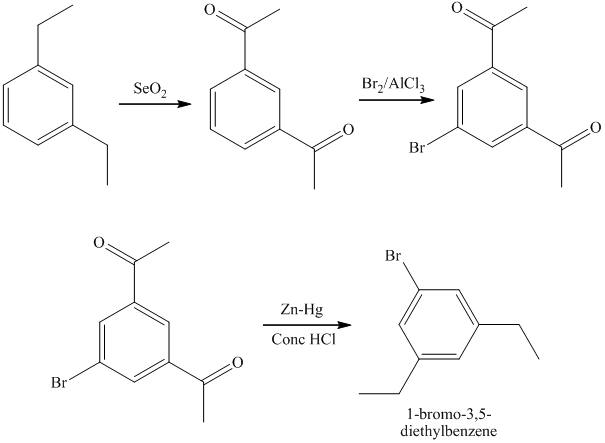
h) The synthesis of
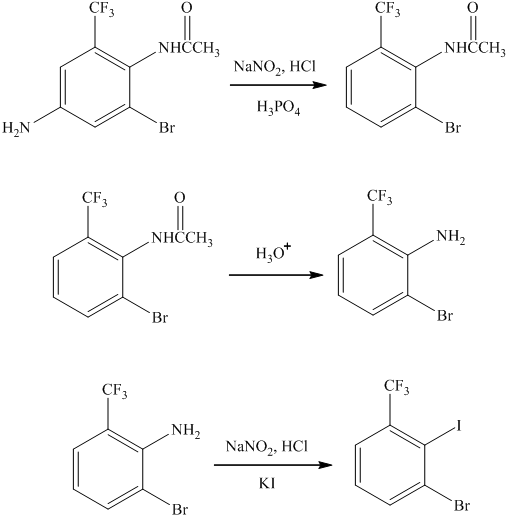
i) The synthesis of the desired compound from its starting material is shown below.
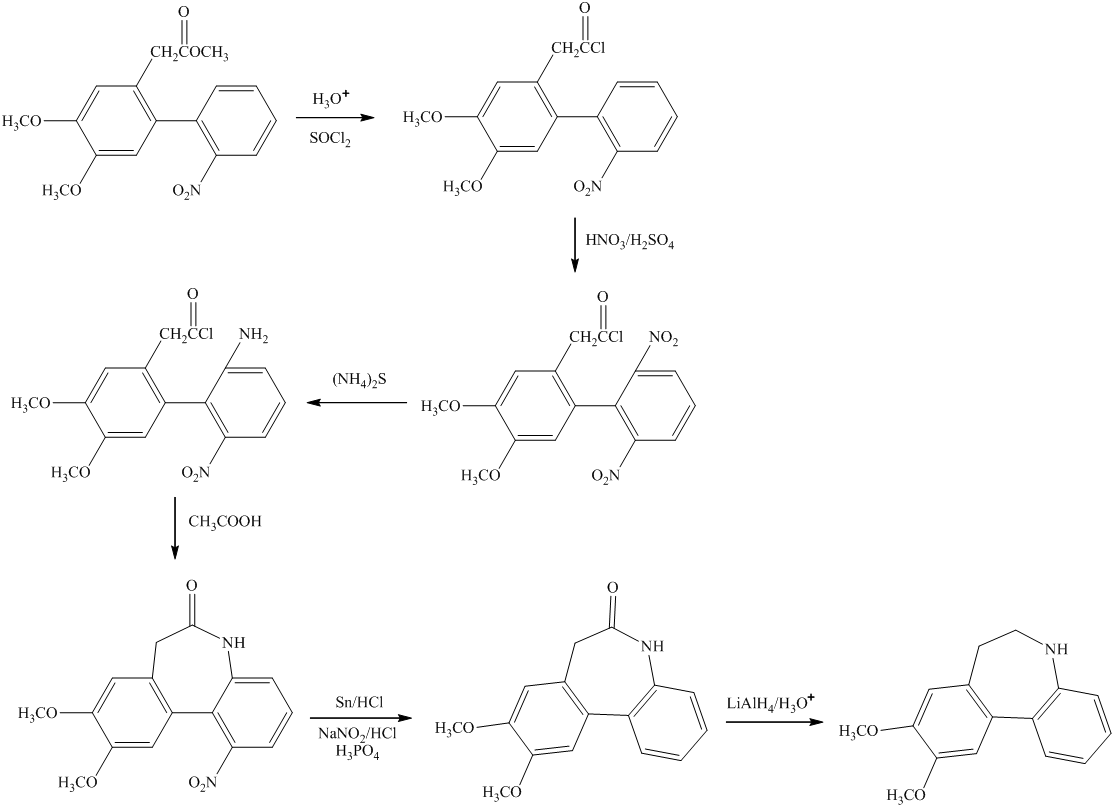
Explanation of Solution
a)
The preparation of

b)
In the preparation of
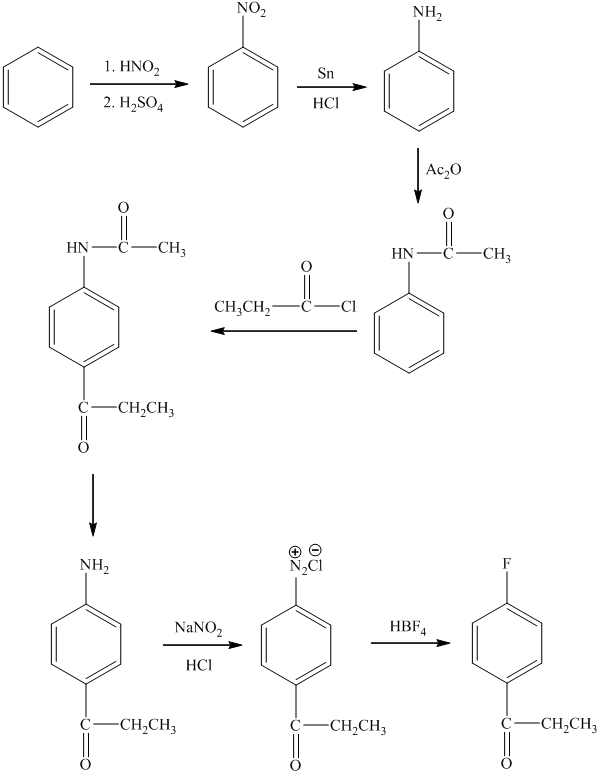
c)
In the preparation of
In the next step, further nitration takes place followed by the addition of
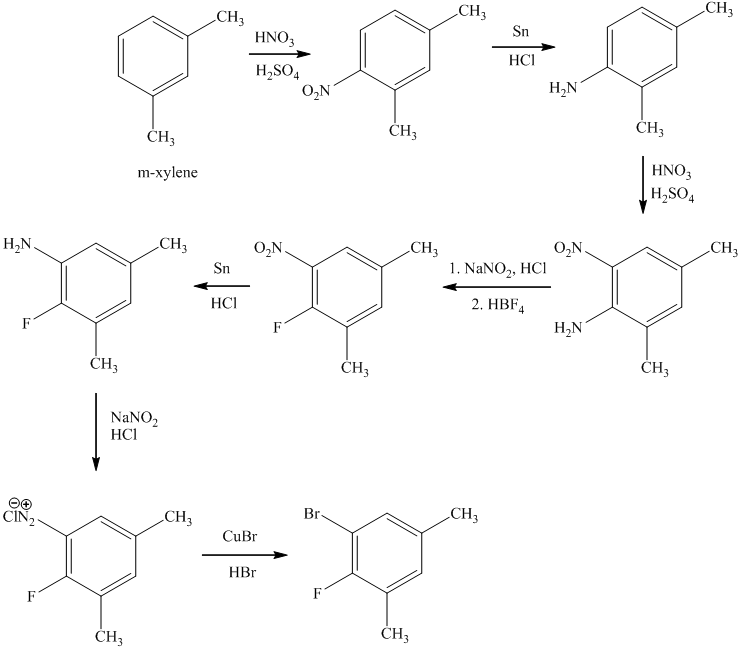
d)
In the first step of the reaction, the starting material reacts with
In the next step, the resulting compound reacts with
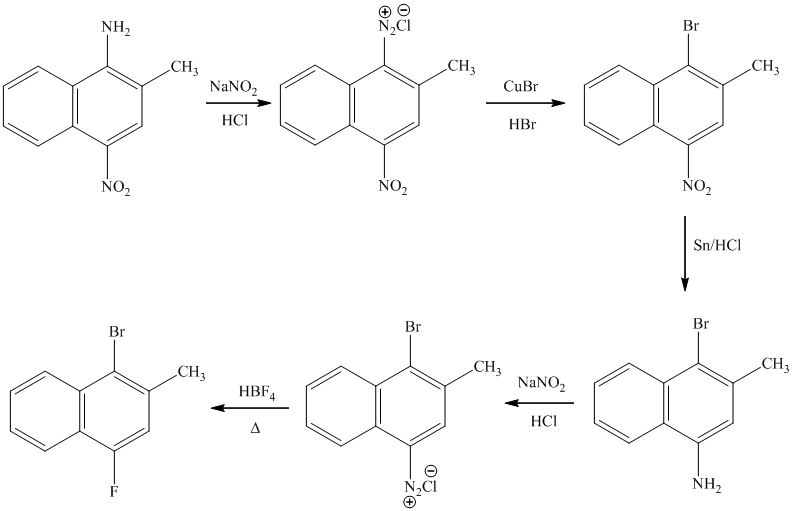
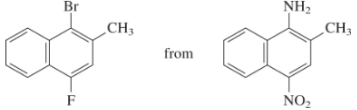
e)
The preparation of
In the next step, the resulting compound reacts with
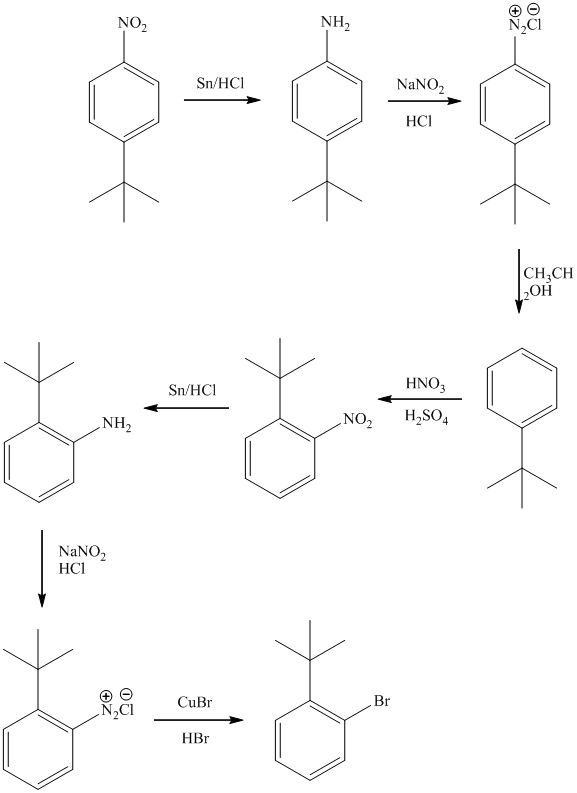
f)
The preparation of
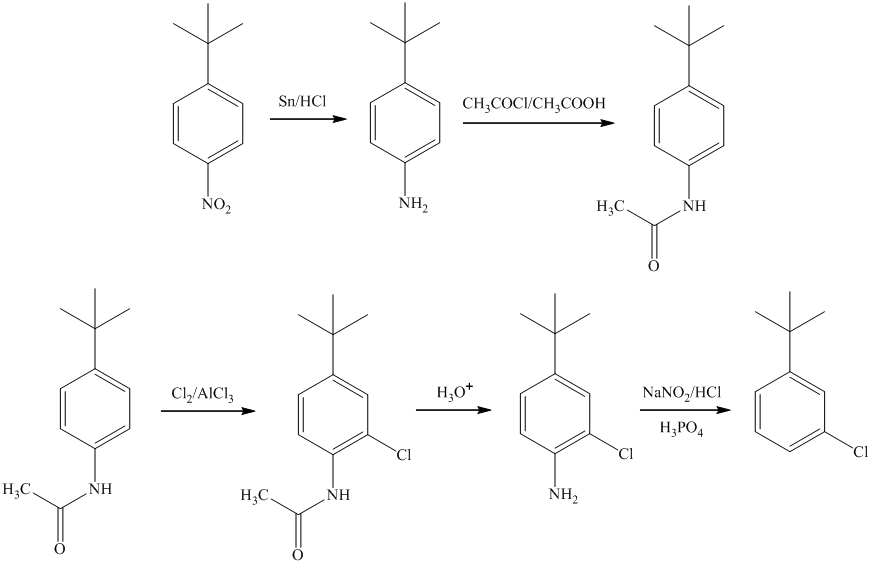
g)
In the synthesis of
In the final step, reduction of ketone occurs to yield the final product
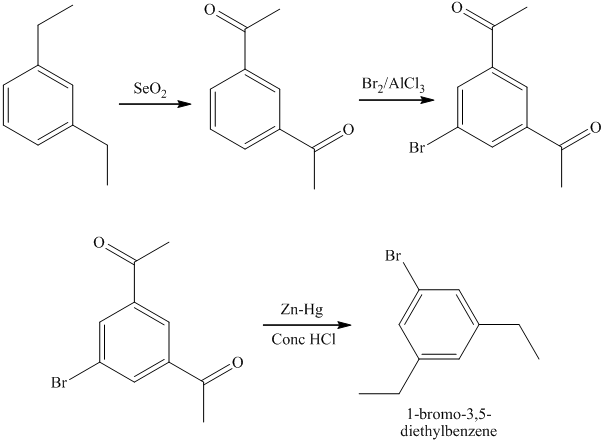
h)
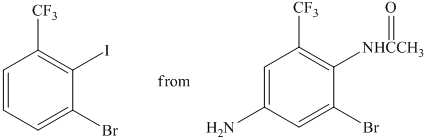
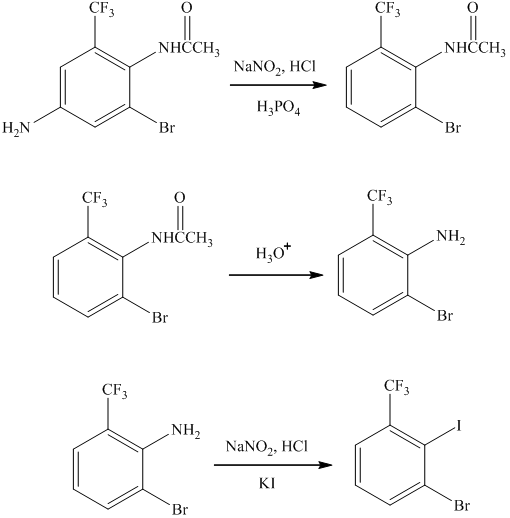
The preparation of
The synthesis of
i)
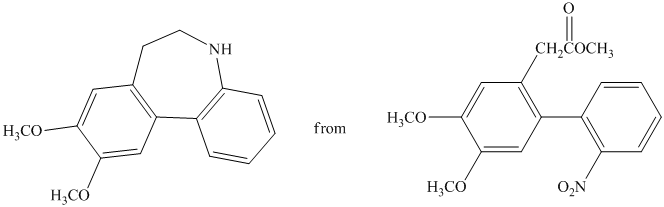
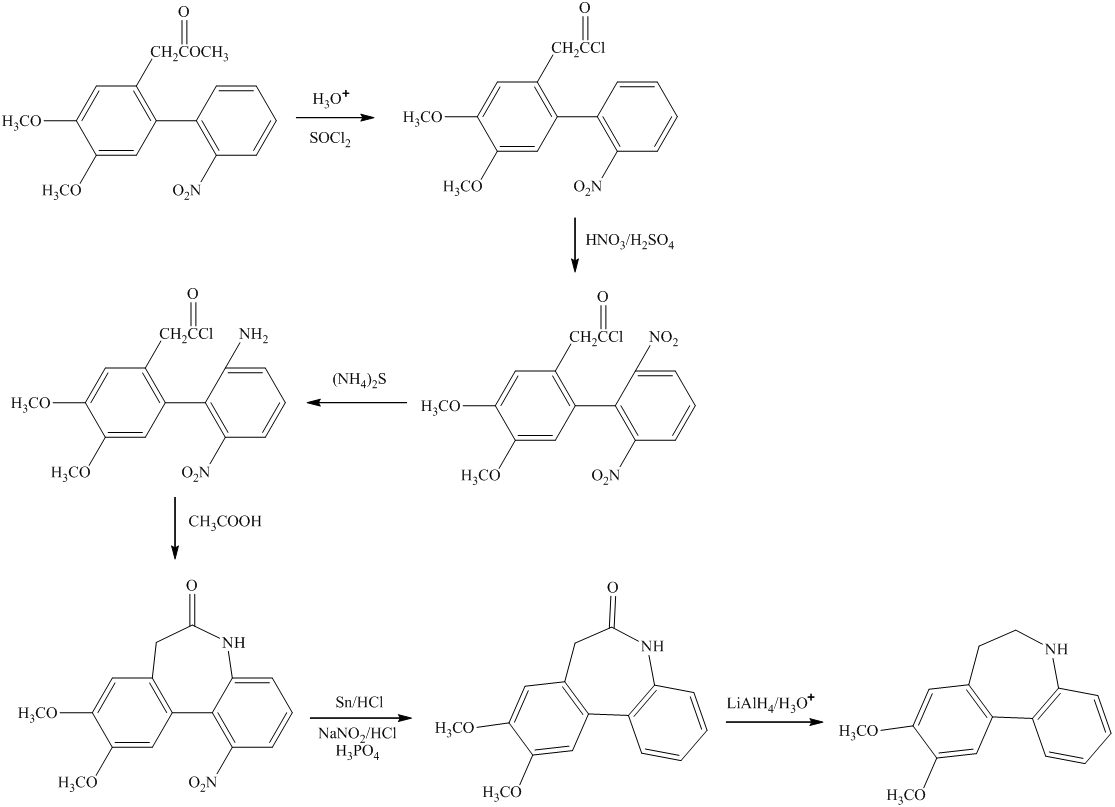
In this conversion, the formation of seven-membered ring present between two benzene rings occurs. Lithium aluminum hydride and sodium borohydride are strong reducing agents. The synthesis of the desired compound from its starting material is shown below.
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





