
Interpretation: Classification of nitrogen atom in spermine as primary, secondary or tertiary
Concept Introduction:
If nitrogen atom is attached to two hydrogen atoms in a compound, it is called primary amine. If nitrogen atom is attached to one hydrogen atoms in a compound, it is called secondary amine. If nitrogen atom is attached to three alkyl groups and no hydrogen atoms in a compound, it is called tertiary amine.
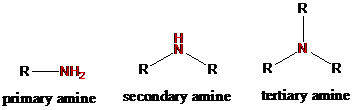
To find: Classify the nitrogen atom in spermine as primary, secondary or tertiary amine
Categorize the nitrogen atom as primary amine in spermine
Answer to Problem 33PP
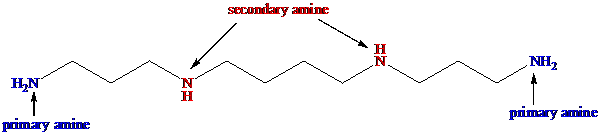
Explanation of Solution
Categorize the nitrogen atom as secondary amine in spermine
The given compound, spermine contains totally four nitrogen atoms. Among them, two of each nitrogen atom is connected with one hydrogen atom. So, it belongs to secondary amine.
Therefore, classification of nitrogen atom in spermine as primary, secondary or tertiary amine is
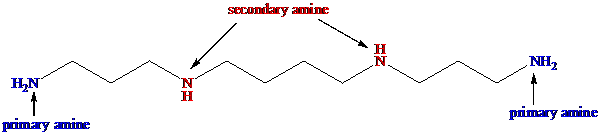
Conclusion
Classification of nitrogen atom in spermine as primary, secondary or tertiary amine should be done by using the concept of number of hydrogen atoms attached to nitrogen atom.
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY 1 TERM ACCESS
- Steps and explanation please.arrow_forwardHow could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forward
- Provide the unknown for the given dataarrow_forwardProvide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





