
Concept explainers
Interpretation:
Effect of hydrophobic interactions on the tertiary structure of proteins must be explained.
Concept introduction:
Proteins are biological
Amino acids are molecules that contain both amino group and
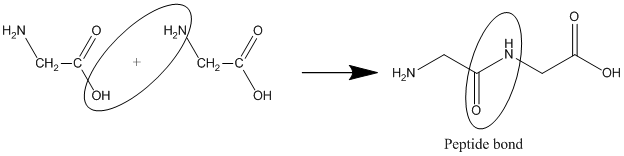
Structure of proteins plays a very important role in their function. Proteins are very complex in structure. Structure of protein is studied in four levels: Primary, Secondary, Tertiary and Quaternary structure.
Primary structure:
Primary structure of a protein is the sequence of amino acids in each polypeptide chain that make up the protein. The ultimate structure of protein depends on this sequence.
Secondary Structure:
The peptide backbone of polypeptide chain folds onto itself due to interactions between amino and carboxylic acid residues in the peptide backbone. This folding of polypeptide chains give proteins a unique shape, this makes the secondary structure of proteins.
Two kinds of shapes are formed in the secondary structure of proteins:
- α-Helix: The backbone folds itself to form a helical structure. Hydrogen bonds are formed with the chain.
- ß-Pleated sheet: The polypeptide chains are stacked side by side. The outer N-H and C=O form intermolecular hydrogen bonds and give a very rigid structure. These hydrogen bonds are formed between neighboring polypeptide chains unlike α-Helix.
Tertiary Structure:
The overall 3-Dimensional structure of a protein formed when regions in secondary structure fold together, is called the tertiary structure of a protein. The tertiary structure of a protein is primarily due to interactions between the side chains of the polypeptide chains or the side chains in the backbone of the polypeptide.
The interactions between the side chains include: hydrogen bonding, ionic interactions, dipole-dipole interactions and London dispersion forces. Another important interaction that makes up the tertiary structure of proteins are hydrophobic interactions between the hydrophobic r groups of side chain of amino acids.
One special kind of covalent bond is also involved in forming the tertiary structure of proteins that is the disulfide bond formed between the -SH residues of cysteine.
Quaternary Structure:
When proteins contain more than one polypeptide chain, the final arrangement of each polypeptide subunit is known as the quaternary structure. The same kinds of interactions that make the tertiary structure are also involved in forming the quaternary structure.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Introduction to General, Organic and Biochemistry
- 2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forwardIndicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forwardWrite chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward
- 2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forwardPlease help me solve number 2arrow_forwardChoose the best reagents to complete the following reaction. 오 Na2Cr2O7 H2SO4, H2O Problem 22 of 35 A Na2Cr2O7 H2SO4, H2O H2/Pt B pressure OH 1. NaBH4 C 2. H3O+ D DMP (Dess-Martin Periodinane) CH2Cl2 CrO3 Done Dramabana_Minor Submitarrow_forward
- Indicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-cyclopentyl-2-methyl-1,3-dioxolane with H3O+. Draw the structures of the compounds.arrow_forward
- Question 4 For the molecule shown below, (7 marks): A) Sketch the Newman projection for the view looking along the bond from the perspective of the arrow. B) Then, draw the Newman projection for each 60° rotation along the bond until it returns to the starting point. C) Clearly indicate which Newman projection is the one we see in the structure shown below, and clearly indicate which Newman projection is the highest in energy and which is the lowest in energy. H H Me 'H Me Mearrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 'N' 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardSubmit Problem 3 of 10 Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. O 'N' NH 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





