
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 22.11, Problem 22.4P
Interpretation Introduction
Interpretation:
The non-covalent interaction occurring between the side chains of arginine and glutamic acid should be explained.
Concept Introduction:
Amino acids are class of organic conpounds containing a carboxyl and an amino group. There are total essential amino acids in the nature. These are essential amino acids because they are not formed inside the body and they are required in the diet. The non-essential amino acids are synthesized by the body thus, they are not required in the diet. The molecular formula of a general amino acid is as follows:
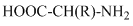
The amino and carboxyl group is same for all amino acid only the difference is in the r group.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which does NOT describe a mole? A. a unit used to count particles directly, B. Avogadro’s number of molecules of a compound, C. the number of atoms in exactly 12 g of pure C-12, D. the SI unit for the amount of a substance
5
What would the complete ionic reaction be if aqueous solutions of potassium sulfate and barium acetate were mixed?
ed
of
Select one:
O a
2 K SO4 + Ba2 +2 C₂H3O21
K+SO4 + Ba2+ + 2 C2H3O21
K+SO42 + Ba2 +2 C2H3O2
BaSO4 +2 K+ + 2 C2H3O
estion
Ob.
O c.
Od.
2 K SO4 +Ba2 +2 C₂H₂O₂
BaSO4 + K+ + 2 C2H3O
BaSO4 + K + 2 C2H301
→Ba² +SO42 +2 KC2H3O
s page
(28 pts.) 7. Propose a synthesis for each of the following transformations. You must include the
reagents and product(s) for each step to receive full credit. The number of steps is provided.
(OC 4)
4 steps
4 steps
OH
b.
Chapter 22 Solutions
Introduction to General, Organic and Biochemistry
Ch. 22.4 - Problem 22-1 Show how to form the dipeptide...Ch. 22.10 - Problem 22-2 What is the oxidation number (the...Ch. 22.10 - Prob. 22.3PCh. 22.11 - Prob. 22.4PCh. 22 - 22-5 What are the functions of (a) ovalbumin and...Ch. 22 - 22-6 The members of which class of proteins are...Ch. 22 - 22-7 What is the function of an immunoglobulin?Ch. 22 - Prob. 22.8PCh. 22 - 22-9 What is the difference in structure between...Ch. 22 - 22-10 Classify the following amino acids as...
Ch. 22 - 22-11 Which amino acid has the highest percentage...Ch. 22 - Prob. 22.12PCh. 22 - Prob. 22.13PCh. 22 - Prob. 22.14PCh. 22 - Prob. 22.15PCh. 22 - 22-16 Which amino acids in Table 22-1 have more...Ch. 22 - 22-17 What are the similarities and differences in...Ch. 22 - 22-18 Draw the structures of L- and D-valine.Ch. 22 - Prob. 22.19PCh. 22 - 22-20 Show how alanine, in solution at its...Ch. 22 - 22-21 Explain why an amino acid cannot exist in an...Ch. 22 - 22-22 Draw the structure of valine at pH 1 and at...Ch. 22 - Prob. 22.23PCh. 22 - 22-24 Draw the most predominant form of histidine...Ch. 22 - 22-25 Draw the most predominant form of lysine at...Ch. 22 - Prob. 22.26PCh. 22 - 22-27 Show by chemical equations how alanine and...Ch. 22 - 22-28 A tetrapeptide is abbreviated as DPKH. Which...Ch. 22 - 22-29 Draw the structure of a tripeptide made of...Ch. 22 - 22-30 (a) Use the three-letter abbreviations to...Ch. 22 - 22-31 A polypeptide chain is made of alternating...Ch. 22 - Prob. 22.32PCh. 22 - 22-33 Which of the three functional groups on...Ch. 22 - Prob. 22.34PCh. 22 - 22-35 Why is histidine considered a basic amino...Ch. 22 - Prob. 22.36PCh. 22 - Prob. 22.37PCh. 22 - 22-38 Why does proline not absorb light at 280 nm?Ch. 22 - Prob. 22.39PCh. 22 - Prob. 22.40PCh. 22 - Prob. 22.41PCh. 22 - 22-42 (a) How many atoms of the peptide bond lie...Ch. 22 - 22-43 (a) Draw the structural formula of the...Ch. 22 - 22-44 How can a protein act as a buffer?Ch. 22 - 22-45 Proteins are least soluble at their...Ch. 22 - 22-46 How many different tripeptides can be made...Ch. 22 - 22-47 How many different tetrapeptides can be made...Ch. 22 - 22-48 How many amino acid residues in the A chain...Ch. 22 - 22-49 Based on your knowledge of the chemical...Ch. 22 - 22-50 Is a random coil a (a) primary, (b)...Ch. 22 - 22-51 Decide whether the following structures that...Ch. 22 - Prob. 22.52PCh. 22 - 22-53 Do iron and zinc ions play role in protein...Ch. 22 - Prob. 22.54PCh. 22 - 22-55 Consider the coordination compound Fe(CO)5...Ch. 22 - Prob. 22.56PCh. 22 - 22-57 Consider the coordination compound...Ch. 22 - Prob. 22.58PCh. 22 - 22-59 What is the effect of salt bridges on the...Ch. 22 - Prob. 22.60PCh. 22 - 22-61 Polyglutamic acid (a polypeptide chain made...Ch. 22 - 22-62 Distinguish between intermolecular and...Ch. 22 - 22-63 Identify the primary, secondary, and...Ch. 22 - 22-64 If both cysteine residues on the B chain of...Ch. 22 - 22-65 (a) What is the difference in the quaternary...Ch. 22 - Prob. 22.66PCh. 22 - Prob. 22.67PCh. 22 - Prob. 22.68PCh. 22 - Prob. 22.69PCh. 22 - Prob. 22.70PCh. 22 - 22-71 Which amino acid side chain is most...Ch. 22 - 22-72 What does the reducing agent do in...Ch. 22 - 22-73 Silver nitrate is sometimes put into the...Ch. 22 - 22-74 Why do nurses and physicians use 70% alcohol...Ch. 22 - 22-75 (Chemical Connections 22A) Why must some...Ch. 22 - Prob. 22.76PCh. 22 - Prob. 22.77PCh. 22 - Prob. 22.78PCh. 22 - Prob. 22.79PCh. 22 - Prob. 22.80PCh. 22 - Prob. 22.81PCh. 22 - 22-82 (Chemical Connections 22H) How does the...Ch. 22 - Prob. 22.83PCh. 22 - 22-84 How many different dipeptides can be made...Ch. 22 - 22-85 Denaturation is usually associated with...Ch. 22 - Prob. 22.86PCh. 22 - Prob. 22.87PCh. 22 - Prob. 22.88PCh. 22 - 22-89 What kind of noncovalent interaction occurs...Ch. 22 - Prob. 22.90PCh. 22 - 22-91 Which amino acid does not rotate the plane...Ch. 22 - 22-92 Write the expected products of the acid...Ch. 22 - 22-93 What charges are on aspartic acid at pH 2.0?Ch. 22 - Prob. 22.94PCh. 22 - Prob. 22.95PCh. 22 - Prob. 22.96PCh. 22 - 22-97 Gelatin is derived from collagen by...Ch. 22 - Prob. 22.98PCh. 22 - Prob. 22.99PCh. 22 - Prob. 22.100PCh. 22 - 22-101 Using what you know about protein...Ch. 22 - Prob. 22.102PCh. 22 - Prob. 22.103PCh. 22 - 22-104 Why is collagen not a very good source of...Ch. 22 - Prob. 22.105P
Knowledge Booster
Similar questions
- LTS Solid: AT=Te-Ti Trial 1 Trial 2 Trial 3 Average ΔΗ Mass water, g 24.096 23.976 23.975 Moles of solid, mol 0.01763 001767 0101781 Temp. change, °C 2.9°C 11700 2.0°C Heat of reaction, J -292.37J -170.473 -193.26J AH, kJ/mole 16.58K 9.647 kJ 10.85 kr 16.58K59.64701 KJ mol 12.35k Minimum AS, J/mol K 41.582 mol-k Remember: q = mCsAT (m = mass of water, Cs=4.184J/g°C) & qsin =-qrxn & Show your calculations for: AH in J and then in kJ/mole for Trial 1: qa (24.0969)(4.1845/g) (-2.9°C)=-292.37J qsin = qrxn = 292.35 292.37J AH in J = 292.375 0.2923kJ 0.01763m01 =1.65×107 AH in kJ/mol = = 16.58K 0.01763mol mol qrx Minimum AS in J/mol K (Hint: use the average initial temperature of the three trials, con Kelvin.) AS=AHIT (1.65×10(9.64×103) + (1.0 Jimaiarrow_forwardFor the compound: C8H17NO2 Use the following information to come up with a plausible structure: 8 This compound has "carboxylic acid amide" and ether functional groups. The peaks at 1.2ppm are two signals that are overlapping one another. One of the two signals is a doublet that represents 6 hydrogens; the other signal is a quartet that represents 3 hydrogens.arrow_forwardVnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest bolling point, choose 2 next to the substance with the next highest boiling point, and so on. substance C D chemical symbol, chemical formula or Lewis structure. CH,-N-CH, CH, H H 10: H C-C-H H H H Cale H 10: H-C-C-N-CH, Bri CH, boiling point (C) Сен (C) B (Choosearrow_forward
- Please help me find the 1/Time, Log [I^-] Log [S2O8^2-], Log(time) on the data table. With calculation steps. And the average for runs 1a-1b. Please help me thanks in advance. Will up vote!arrow_forwardQ1: Answer the questions for the reaction below: ..!! Br OH a) Predict the product(s) of the reaction. b) Is the substrate optically active? Are the product(s) optically active as a mix? c) Draw the curved arrow mechanism for the reaction. d) What happens to the SN1 reaction rate in each of these instances: 1. Change the substrate to Br "CI 2. Change the substrate to 3. Change the solvent from 100% CH3CH2OH to 10% CH3CH2OH + 90% DMF 4. Increase the substrate concentration by 3-fold.arrow_forwardExperiment 27 hates & Mechanisms of Reations Method I visual Clock Reaction A. Concentration effects on reaction Rates Iodine Run [I] mol/L [S₂082] | Time mo/L (SCC) 0.04 54.7 Log 1/ Time Temp Log [ ] 13,20] (time) / [I] 199 20.06 23.0 30.04 0.04 0.04 80.0 22.8 45 40.02 0.04 79.0 21.6 50.08 0.03 51.0 22.4 60-080-02 95.0 23.4 7 0.08 0-01 1970 23.4 8 0.08 0.04 16.1 22.6arrow_forward
- (15 pts) Consider the molecule B2H6. Generate a molecular orbital diagram but this time using a different approach that draws on your knowledge and ability to put concepts together. First use VSEPR or some other method to make sure you know the ground state structure of the molecule. Next, generate an MO diagram for BH2. Sketch the highest occupied and lowest unoccupied MOs of the BH2 fragment. These are called frontier orbitals. Now use these frontier orbitals as your basis set for producing LGO's for B2H6. Since the BH2 frontier orbitals become the LGOS, you will have to think about what is in the middle of the molecule and treat its basis as well. Do you arrive at the same qualitative MO diagram as is discussed in the book? Sketch the new highest occupied and lowest unoccupied MOs for the molecule (B2H6).arrow_forwardQ8: Propose an efficient synthesis of cyclopentene from cyclopentane.arrow_forwardQ7: Use compound A-D, design two different ways to synthesize E. Which way is preferred? Please explain. CH3I ONa NaOCH 3 A B C D E OCH3arrow_forward
- Predict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward(10 pts) The density of metallic copper is 8.92 g cm³. The structure of this metal is cubic close-packed. What is the atomic radius of copper in copper metal?arrow_forwardPredict major product(s) for the following reactions. Note the mechanism(s) of the reactions (SN1, E1, SN2 or E2).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning