
Concept explainers
Interpretation:
The molecular formula and the condensed structural formula for the given organic compounds are to be given.
Concept Introduction:
The molecular formula indicates the total number of atoms involved in a molecule of a substance.
The condensed structural formula of a molecule does not contain any branching above or below. It is a system of writing organic structures in a line of text.
Answer to Problem 1PE
Solution:
a)
b)
c)
Explanation of Solution
a)

The molecular formula of the given organic compound consists of 6 carbon atoms and
The condensed structural formula for the given organic compound contains 6 carbon atoms and
b)
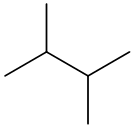
The molecular formula of the given organic compound consists of 6 carbon atoms and
The condensed structural formula for the given organic compound contains 6 carbon atoms and
All carbon and hydrogen atoms are linked together by a single bond and it can be represented as follows:
c)
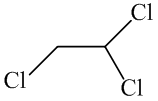
The molecular formula of the given organic compound consists of two carbon atoms,
The condensed structural formula for the given organic compound contains 2 carbon atoms,
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry: The Molecular Nature of Matter
- Indicate the substitutes in one place, if they are a diazonio room.arrow_forwardIndicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Synthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forwardSynthesis of 1-metilbenzotriazole.arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forward
- Identify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forwardWhat is single-point calibration? Provide an example.arrow_forwardDraw the major product formed via an E1 pathway.arrow_forward
- Part 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forwardConsider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


