
(a)
Interpretation:
The ketone or aldehyde that can be used to produce the given compound when treated with
Concept introduction:
Answer to Problem 21.61P
The ketone that can be used to produce the given compound when treated with
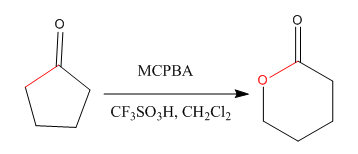
Explanation of Solution
The given compound is:
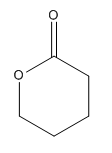
The given compound is an ester that can be produced when a ketone treated with the
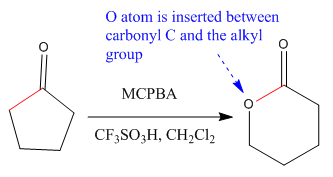
The ketone that can be used to produce the given compound is drawn, removing the oxygen next to the carbonyl C which is inserted when it reacts with
(b)
Interpretation:
The ketone or aldehyde that can be used to produce the given compound when treated with
Concept introduction:
Answer to Problem 21.61P
The aldehyde that can be used to produce the given compound when treated with
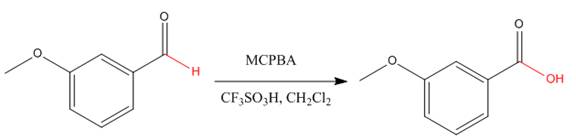
Explanation of Solution
The given compound is:
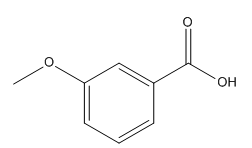
The given compound is an acid that can be produced when a aldehyde is treated with
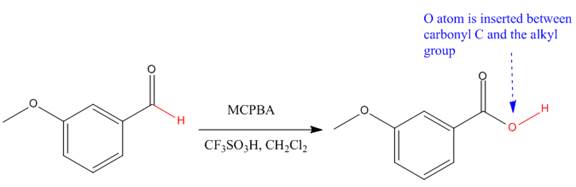
The ketone that can be used to produce the given compound is drawn, removing the oxygen next to the carbonyl C which is inserted when it reacts with
(c)
Interpretation:
The ketone or aldehyde that can be used to produce the given compound when treated with
Concept introduction:
Answer to Problem 21.61P
The ketone that can be used to produce the given compound when it is treated with
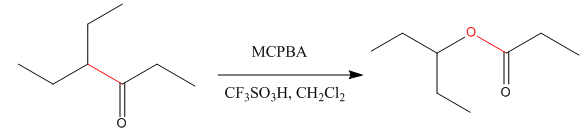
Explanation of Solution
The given compound is:
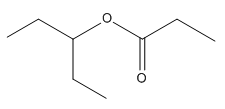
The given compound is an ester that can be produced when a ketone is treated with
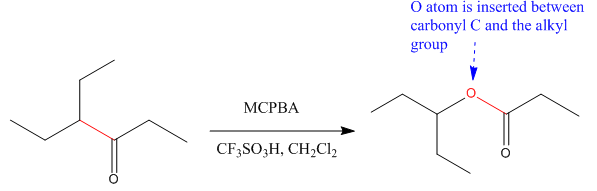
The ketone that can be used to produce the given compound is drawn, removing the oxygen next to the carbonyl C which inserted when it react with
(d)
Interpretation:
The ketone or aldehyde that can be used to produce the given compound when treated with
Concept introduction:
Answer to Problem 21.61P
The ketone that can be used to produce the given compound when it is treated with
Explanation of Solution
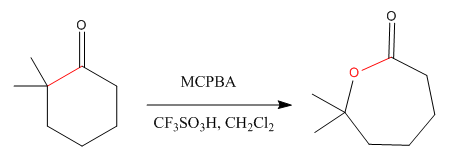
The given compound is:
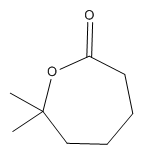
The given compound is an ester that can be produced when a ketone is treated with
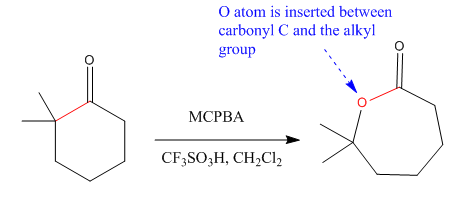
The ketone that can be used to produce the given compound is drawn, removing the oxygen next to the carbonyl C which is inserted when it reacts with
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Assign these protonarrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forward
- Could you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forwardCan you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forward
- Part 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





