
Concept explainers
Give the IUPAC name for each compound.
a. 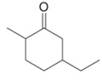 c.
c. 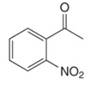 e.
e. 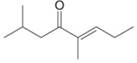
b. 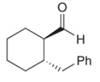 d.
d. 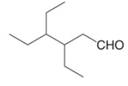 f.
f. 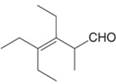
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
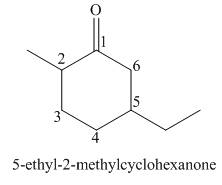
Figure 1
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
Figure 2
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(c)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
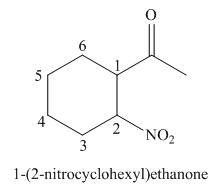
Figure 3
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(d)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
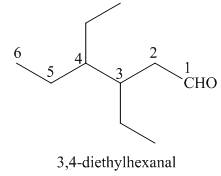
Figure 4
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(e)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of eight
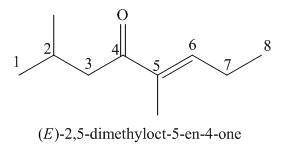
Figure 5
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
(f)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.43P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
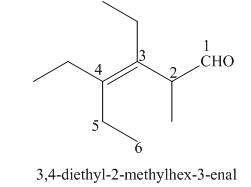
Figure 6
Thus, the IUPAC name for the given compound is
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 21 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Additional Science Textbook Solutions
Loose Leaf For Integrated Principles Of Zoology
Biology: Concepts and Investigations
Brock Biology of Microorganisms (15th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Identify the unknown compound from its IR and proton NMR spectra. C4H6O: 'H NMR: 82.43 (1H, t, J = 2 Hz); 8 3.41 (3H, s); 8 4.10 (2H, d, J = 2 Hz) IR: 2125, 3300 cm¹ The C4H6O compound liberates a gas when treated with C2H5 MgBr. Draw the unknown compound. Select Draw с H Templates Morearrow_forwardPlease help with number 6 I got a negative number could that be right?arrow_forward1,4-Dimethyl-1,3-cyclohexadiene can undergo 1,2- or 1,4-addition with hydrogen halides. (a) 1,2-Addition i. Draw the carbocation intermediate(s) formed during the 1,2-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,2-addition product formed during the reaction in (i)? (b) 1,4-Addition i. Draw the carbocation intermediate(s) formed during the 1,4-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,4-addition product formed from the reaction in (i)? (c) What is the kinetic product from the reaction of one mole of hydrobromic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (d) What is the thermodynamic product from the reaction of one mole of hydrobro-mic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (e) What major product will result when 1,4-dimethyl-1,3-cyclohexadiene is treated with one mole of hydrobromic acid at - 78 deg * C ? Explain your reasoning.arrow_forward
- Give the product of the bimolecular elimination from each of the isomeric halogenated compounds. Reaction A Reaction B. КОВ CH₂ HotBu +B+ ко HOIBU +Br+ Templates More QQQ Select Cv Templates More Cras QQQ One of these compounds undergoes elimination 50x faster than the other. Which one and why? Reaction A because the conformation needed for elimination places the phenyl groups and to each other Reaction A because the conformation needed for elimination places the phenyl groups gauche to each other. ◇ Reaction B because the conformation needed for elimination places the phenyl groups gach to each other. Reaction B because the conformation needed for elimination places the phenyl groups anti to each other.arrow_forwardFive isomeric alkenes. A through each undergo catalytic hydrogenation to give 2-methylpentane The IR spectra of these five alkenes have the key absorptions (in cm Compound Compound A –912. (§), 994 (5), 1643 (%), 3077 (1) Compound B 833 (3), 1667 (W), 3050 (weak shoulder on C-Habsorption) Compound C Compound D) –714 (5), 1665 (w), 3010 (m) 885 (3), 1650 (m), 3086 (m) 967 (5), no aharption 1600 to 1700, 3040 (m) Compound K Match each compound to the data presented. Compound A Compound B Compound C Compound D Compoundarrow_forward7. The three sets of replicate results below were accumulated for the analysis of the same sample. Pool these data to obtain the most efficient estimate of the mean analyte content and the standard deviation. Lead content/ppm: Set 1 Set 2 Set 3 1. 9.76 9.87 9.85 2. 9.42 9.64 9.91 3. 9.53 9.71 9.42 9.81 9.49arrow_forward
- Draw the Zaitsev product famed when 2,3-dimethylpentan-3-of undergoes an El dehydration. CH₂ E1 OH H₁PO₁ Select Draw Templates More QQQ +H₂Oarrow_forwardComplete the clean-pushing mechanism for the given ether synthesia from propanol in concentrated sulfurica140°C by adding any mining aloms, bands, charges, nonbonding electron pairs, and curved arrows. Draw hydrogen bonded to cayan, when applicable. ore 11,0 HPC Step 1: Draw curved arrows Step 2: Complete the intend carved Q2Q 56 QQQ Step 3: Complete the intermediate and add curved Step 4: Modify the structures to draw the QQQ QQQarrow_forward6. In an experiment the following replicate set of volume measurements (cm3) was recorded: (25.35, 25.80, 25.28, 25.50, 25.45, 25.43) A. Calculate the mean of the raw data. B. Using the rejection quotient (Q-test) reject any questionable results. C. Recalculate the mean and compare it with the value obtained in 2(a).arrow_forward
- A student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T G OH де OH This transformation can't be done in one step.arrow_forwardMacmillan Leaming Draw the major organic product of the reaction. 1. CH3CH2MgBr 2. H+ - G Select Draw Templates More H о QQarrow_forwardDraw the condensed structure of 3-hydroxy-2-butanone. Click anywhere to draw the first atom of your structure.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



