
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.15P
Draw the product of each reaction.
a.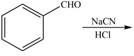 b.
b. 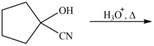
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
First image: Why can't the molecule C be formed in those conditions
Second image: Synthesis for lactone C
its not an exam
First image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion
Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary
First image: I have to explain why the molecule C is never formed in those conditions.
Second image: I have to propose a synthesis for the lactone A
Chapter 21 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 21 - Rank the following compounds in order of...Ch. 21 - Prob. 21.2PCh. 21 - Give the IUPAC name for each aldehyde.Ch. 21 - Prob. 21.4PCh. 21 - Give the IUPAC name for each ketone.Ch. 21 - Prob. 21.6PCh. 21 - Prob. 21.7PCh. 21 - The boiling point of is significantly higher than...Ch. 21 - Which carbonyl group in each pair absorbs at a...Ch. 21 - Problem 21.10 Draw the structure of all...
Ch. 21 - Prob. 21.11PCh. 21 - Prob. 21.12PCh. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Problem 21.15 Draw the product of each...Ch. 21 - Prob. 21.16PCh. 21 - Problem 21.17 Draw the products of the following...Ch. 21 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 21 - Problem 21.19 Draw the products (including...Ch. 21 - Problem 21.20 What starting materials are needed...Ch. 21 - Prob. 21.21PCh. 21 - Problem 21.22 The product formed when reacts with...Ch. 21 - Prob. 21.23PCh. 21 - Prob. 21.24PCh. 21 - Prob. 21.25PCh. 21 - Prob. 21.26PCh. 21 - Prob. 21.27PCh. 21 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.29 Draw the products of each...Ch. 21 - Problem 21.30 Label each compound as an acetal, a...Ch. 21 - Problem 21.31 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.32 Draw the products of each...Ch. 21 - Problem 21.33 Safrole is a naturally occurring...Ch. 21 - Prob. 21.34PCh. 21 - Problem 21.35 How would you use a protecting group...Ch. 21 - Prob. 21.36PCh. 21 - Problem 21.37 Two naturally occurring compounds...Ch. 21 - Problem 21.38 Draw the products of each...Ch. 21 - Prob. 21.39PCh. 21 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 21 - 21.41 Rank the following compounds in order of...Ch. 21 - Prob. 21.42PCh. 21 - 21.43 Give the IUPAC name for each compound.
a....Ch. 21 - 21.44 Give the structure corresponding to each...Ch. 21 - Prob. 21.45PCh. 21 - 21.46 Draw the products of each reaction.
a. e....Ch. 21 - Prob. 21.47PCh. 21 - 21.48 Draw all stereoisomers formed in each...Ch. 21 - Prob. 21.49PCh. 21 - What products are formed when each acetal is...Ch. 21 - Prob. 21.51PCh. 21 - Prob. 21.52PCh. 21 - Which compound forms the higher concentration of...Ch. 21 - Prob. 21.54PCh. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Devise a synthesis of each alkene using a Wittig...Ch. 21 - Devise a synthesis of each compound from...Ch. 21 - Prob. 21.60PCh. 21 - Devise a synthesis of each compound from ethanol...Ch. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - 21.64 Draw a stepwise mechanism for the following...Ch. 21 - 21.65 Draw a stepwise mechanism f or the following...Ch. 21 - Prob. 21.66PCh. 21 - 21.67 Draw a stepwise mechanism for each...Ch. 21 - 21.68 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.69PCh. 21 - Prob. 21.70PCh. 21 - Prob. 21.71PCh. 21 - Prob. 21.72PCh. 21 - 21.73 Although the carbonyl absorption of cyclic...Ch. 21 - 21.74 Use the and data to determine the...Ch. 21 - 21.75 A solution of acetone in ethanol in the...Ch. 21 - Compounds A and B have molecular formula ....Ch. 21 - 21.77 An unknown compound C of molecular formula ...Ch. 21 - 21.78 An unknown compound D exhibits a strong...Ch. 21 - Prob. 21.79PCh. 21 - -D-Glucose, a hemiacetal, can be converted to a...Ch. 21 - 21.81 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.82PCh. 21 - 21.83 Draw a stepwise mechanism f or the...Ch. 21 - Prob. 21.84PCh. 21 - Prob. 21.85PCh. 21 - 21.86 Draw stepwise mechanism for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forwardGiven the attached data, provide the drawing for the corresponding structure.arrow_forwardno Ai walkthroughsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY