
Concept explainers
Give the IUPAC name for each compound.
a. 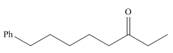 d.
d. 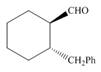 g.
g. 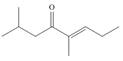
b. 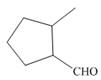 e.
e. 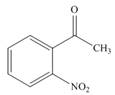 h.
h. 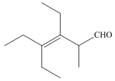
c. 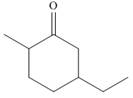 f.
f. 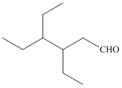
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of eight
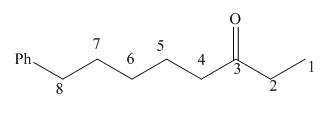
Figure 1
Thus, the IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of the aldehyde. The first step is finding of longest parent chain that contains a carbonyl group. The second step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of five
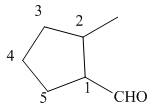
Figure 2
Thus, the IUPAC name for the given compound is
(c)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
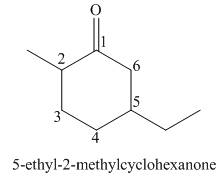
Figure 3
Thus, the IUPAC name for the given compound is
(d)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
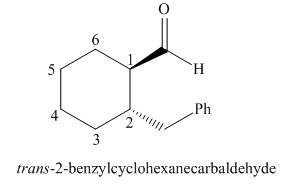
Figure 4
Thus, the IUPAC name for the given compound is
(e)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of six
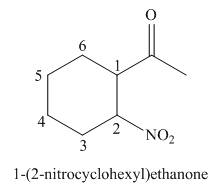
Figure 5
Thus, the IUPAC name for the given compound is
(f)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
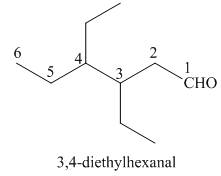
Figure 6
Thus, the IUPAC name for the given compound is
(g)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl carbon. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is a ketone.
One should follow the given steps to give the IUPAC name of a ketone. The first step is finding of longest parent chain that contains a carbonyl group. The second step is changing of -e ending of the parent alkane to the suffix -one. The third step is numbering of chain to give the least number to carbonyl carbon, and using the general rules of nomenclature.
The given structure shows the presence of eight
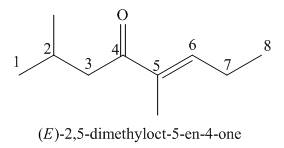
Figure 7
Thus, the IUPAC name for the given compound is
(h)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.42P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is an aldehyde.
One should follow the given steps to give the IUPAC name of an aldehyde. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given structure shows the presence of six
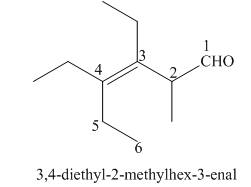
Figure 8
Thus, the IUPAC name for the given compound is
(a) The IUPAC name for the given compound is
(b) The IUPAC name for the given compound is
(c) The IUPAC name for the given compound is
(d) The IUPAC name for the given compound is
(e) The IUPAC name for the given compound is
(f) The IUPAC name for the given compound is
(g) The IUPAC name for the given compound is
(h) The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- If 10 mL of a commercial sodium silicate solution is added, the water required to obtain a 20% solids solution (SiO2+Na2O) is added. Indicate the final grams of Na2SiO3.arrow_forwardPlease help me figure out the mechanism with arrows of the following reactionarrow_forwardOrganic Functional Groups Predicting the reactants or products of acetal hydrolysis termine the structures of the missing organic molecules in the following reaction: H* H* + H₂O Y ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure Explanation Check @2 W Click and drag to start drawing a structure. #4 # 3 LU E % 67 olo 5 66 R T Y & 7 AcGraw Hill LLC. All Rights R Xarrow_forward
- 8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enaminearrow_forwardDraw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forward
- Predict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forwardPredict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forward
- Please draw the structuresarrow_forwardDraw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





