
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
Answer to Problem 21.1P
The structure of
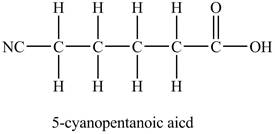
Explanation of Solution
In
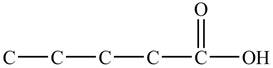
Figure 1
At the fifth carbon, there is a cyano group
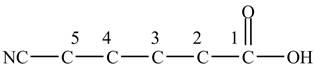
Figure 2
The above skeleton is completed by adding hydrogens as per the tetravalency of carbon as shown below.
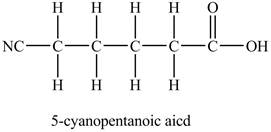
Figure 3
The structure of
(b)
Interpretation:
The structure of isopropyl valerate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of isopropyl valerate is shown below.
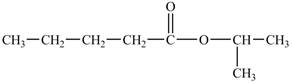
Explanation of Solution
The structure of isopropyl valerate consists of eight carbons, sixteen hydrogen atoms and two oxygen atoms. Isopropyl valerate is a common name of isopropyl pentanoate. It is a five carbon carboxylic acid. The structure of pentanoic acid is shown below.
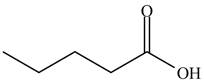
Figure 4
The hydrogen of carboxylic group is replaced by an isopropyl group to form an ester. The structure of isopropyl valerate is shown below.
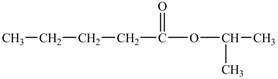
Figure 5
The structure of isopropyl valerate is shown in Figure 5.
(c)
Interpretation:
The structure of ethyl methyl malonate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of ethyl methyl malonate is shown below.
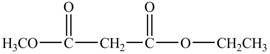
Explanation of Solution
The structure of ethyl methyl malonate consists of a eight carbons, fourteen hydrogen and four oxygen. Ethyl methyl malonate consists of propane dioic acid as parent chain.
The structure of propane dioic acid is shown below.
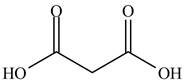
Figure 6
In the above structure, at first position hydrogen is substituted by a methyl group and at the last carboxyl group an ethyl group replaces the hydrogen of the carboxyl group. The structure of ethyl methyl malonate is shown below.
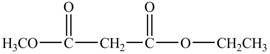
Figure 7
The structure of ethyl methyl malonate is shown in Figure 6.
(d)
Interpretation:
The structure of cyclohexyl acetate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of cyclohexyl acetate is shown below.
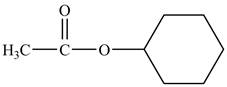
Explanation of Solution
Cyclohexyl acetate consists of a cyclohexane ring and an acetate group. The acetate group is given by the formula
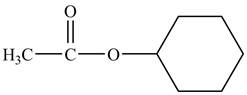
Figure 8
The structure of cyclohexyl acetate is shown in Figure 8
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
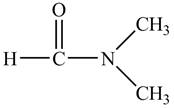
Explanation of Solution
The structure of
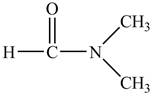
Figure 9
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
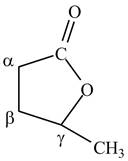
Explanation of Solution
Lactones are cyclic esters. The structure of
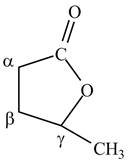
Figure 10
The structure of
(g)
Interpretation:
The structure of glutarimide is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of glutarimide is shown below.
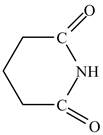
Explanation of Solution
The structure of glutarimide consists of a piperidine ring. There are two carbonyl group present adjacent to the nitrogen group. The structure of a piperidine ring is shown below.
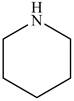
Figure 11
Therefore, the structure of glutarimide is shown below.
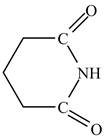
Figure 12
The structure of glutarimide is shown in Figure 12.
(h)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
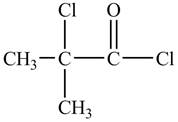
Explanation of Solution
There is carboxyl group present in
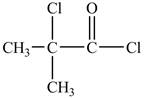
Figure 13
The structure of
(i)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
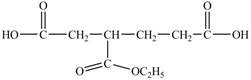
Explanation of Solution
In
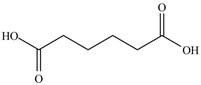
Figure 14
In the above structure there is a ethoxy carbonyl group at the third position. Therefore, the structure of
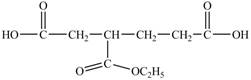
Figure 15
The structure of
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forward
- Potential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forward
- Draw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

