
Interpretation:
Whether an achiral or two equal enantiomeric products would be given has to be predicted in the product of the given reaction and explained with chair like transition state.
Concept Introduction:
Cope-rearrangement:
It is a pericyclic reaction that involves the redistribution of six electrons through the formation of a cyclic transition state from which a
Example with mechanism of cope-arrangement:
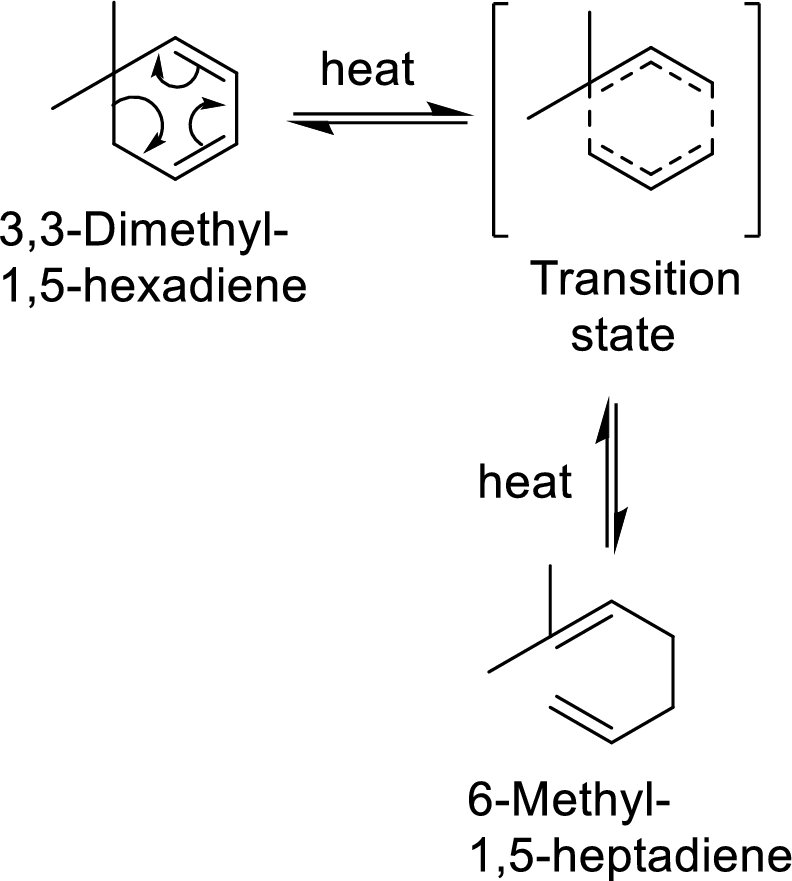
In this mechanism, two pi-bonds and one sigma bond of the reactant molecule has been rearranged and formed two new pi-bonds through a cyclic transition state.
Identification of cope-rearrangement in a
In the cope-rearrangement, the flow of electrons takes place between six bonds that are bonded as
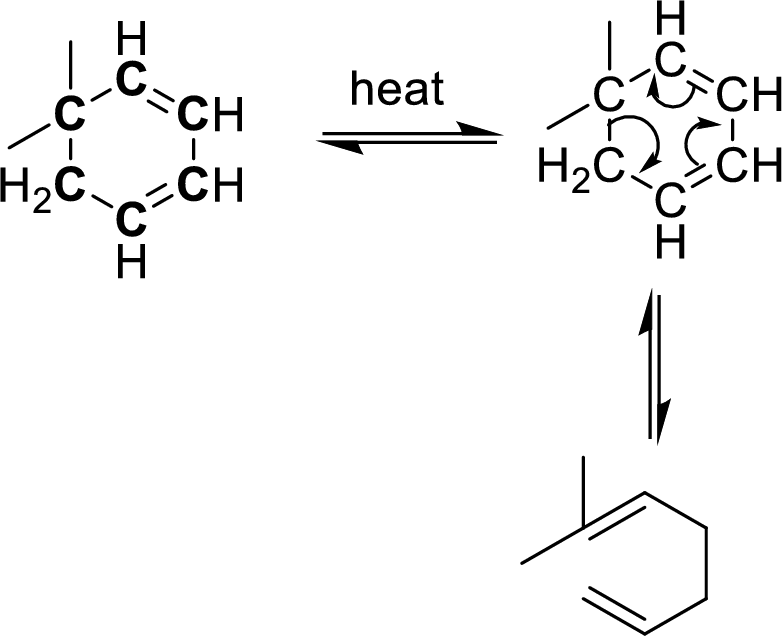
The carbon atoms that are involving in the cope-rearrangement are shown in bold.
Stereochemistry in a product formed:
- • In the product of a
chemical reaction , if a carbon atom has been attached with four different carbon atoms, then it is known as chiral carbon atom or stereocenter in the product. - • The bonds of the
functional groups because of which a new chiral carbon is supposed to form have to be represented in solid wedge bond and hashed-wedge bonds according to the particular enantiomer. - • Racemic mixture is the mixture of two enantiomers in equal proportions.
- • Enantiomers are non-superimposable mirror images.
- • Achiral product is the product in which there won’t be any chiral centre or stereocenter.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

