
Concept explainers
(a)
Interpretation: The structural formula for the Diels-Alder adduct formed by cyclopentadiene has to be drawn.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
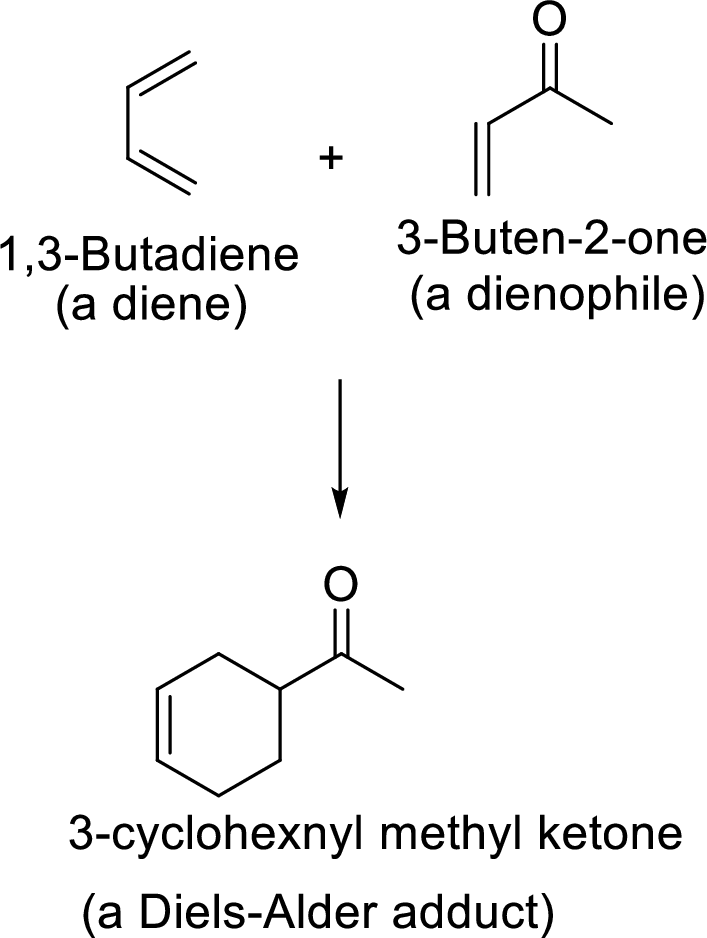
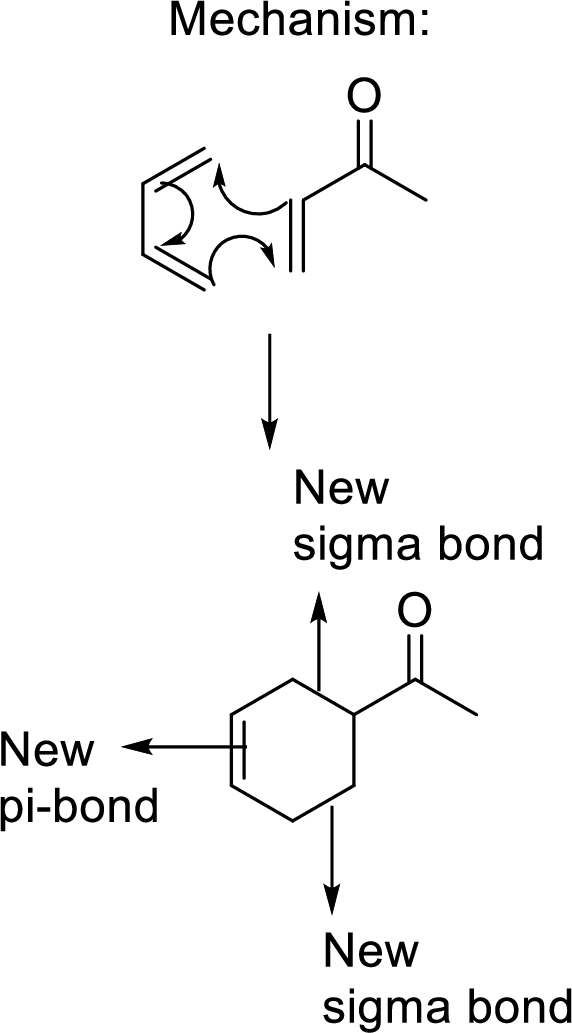
This mechanism shown that three
Diels-Alder reaction to form bicyclic system:
The Diels-Alder adduct formed in the Diels-Alder reaction can also be a bicyclic system which will be obtained when cylopentadiene is used as the diene as shown here:
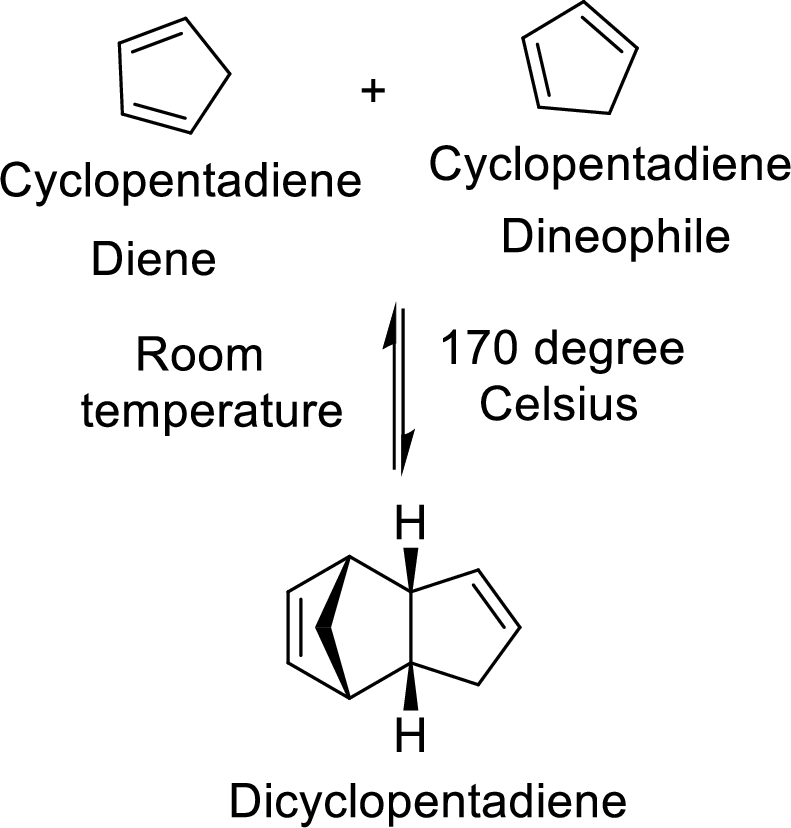
In this reaction, the cylopentadiene acts as both diene and dienophile and formed a bicyclic system. When it is heated to
(a)
Explanation of Solution
The given reaction is:
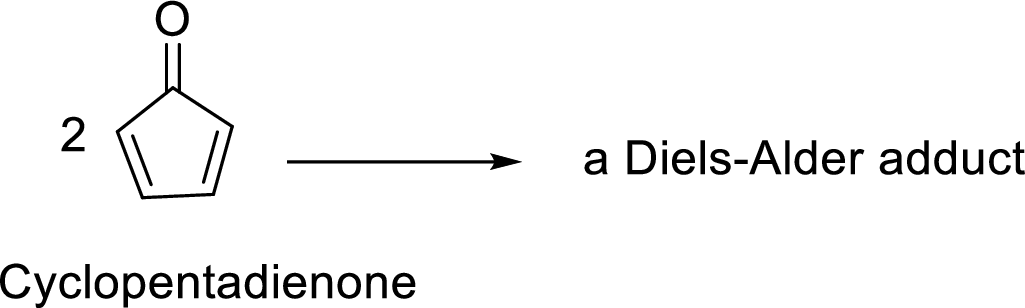
The structural formula for the Diels-Alder adduct can be found using the following mechanism of the given Diels-Alder reaction:
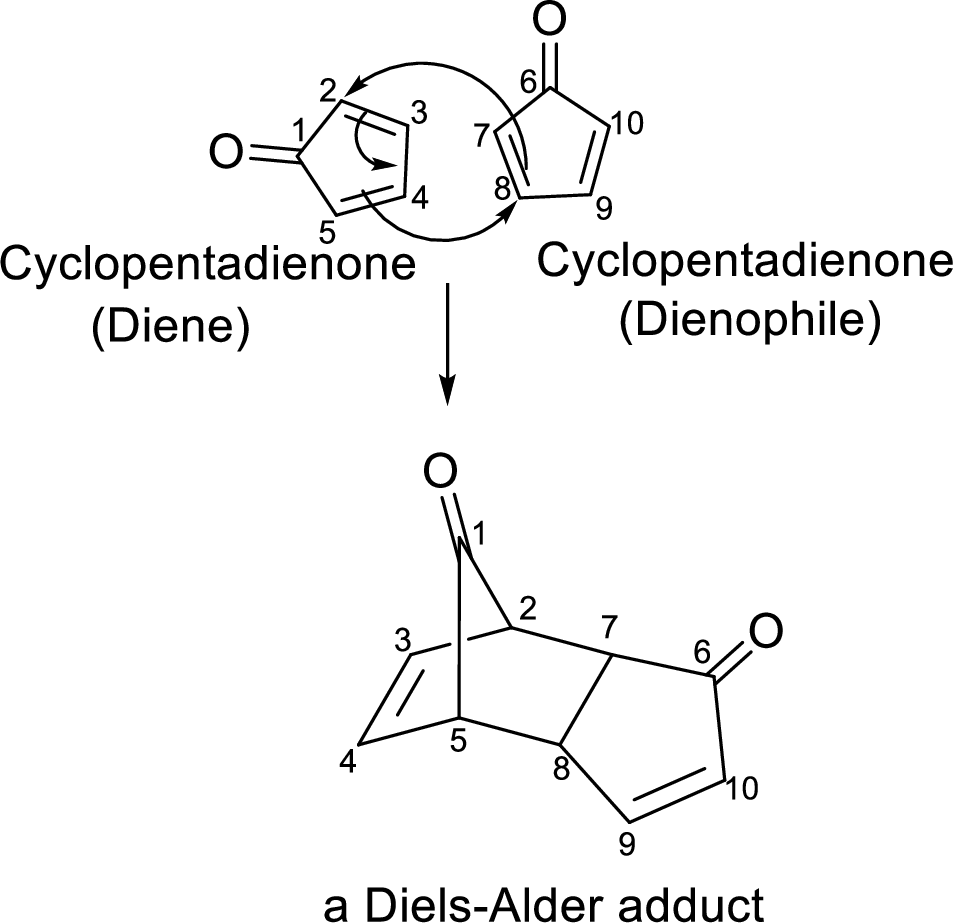
In this Diels-Alder reaction, one molecule of cyclopentadienone acts as a Diene whereas the other molecule of cyclopentadienone acts as a Dienophile. The resulted Diels-Alder adduct is a tricyclic product
(b)
Interpretation: The difference in the stability of the given
Concept Introduction:
Stability based on aromaticity:
The term aromaticity means “extreme stability”. So,
The aromatic compounds and anti-aromatic compounds can be distinguished based on Huckel’s
Huckel’s rule of aromaticity is
If
If
(b)
Answer to Problem 20.44P
The difference in the stability of the given ketones has been accounted as:
Cycloheptatrienone is more stable than cyclopentadienone.
Explanation of Solution
The given ketones are:
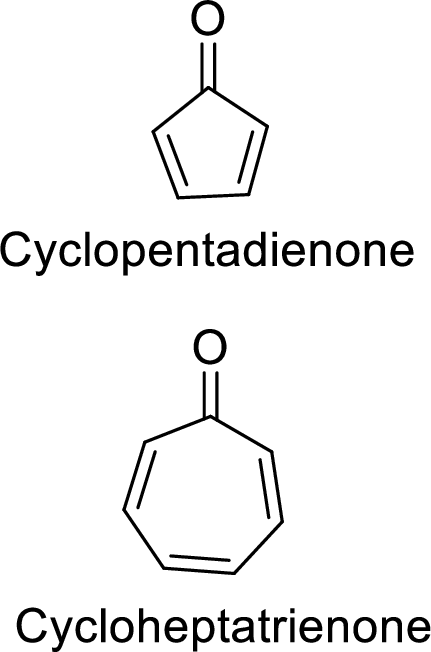
The stability of these two ketones can be distinguished based on aromaticity as discussed below:
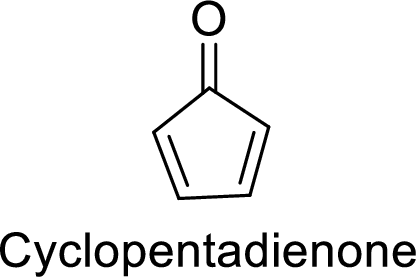
There are two
Huckel’s rule of aromaticity:
Therefore, the given molecule has
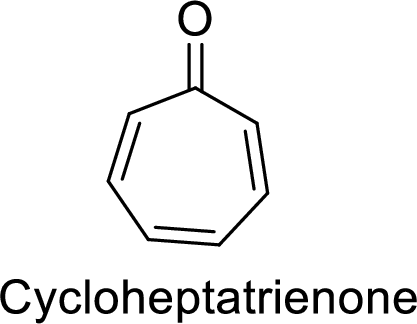
There are three
Huckel’s rule of aromaticity:
Therefore, the given molecule satisfies the Huckel’s rule of aromaticity. So, it is an anti-aromatic compound which means it is a highly stable compound.
Hence, the overall discussion makes it clear that cycloheptatrienone is more stable than cyclopentadienone.
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


