
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.35P
The following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction shown in Problem 20.34.
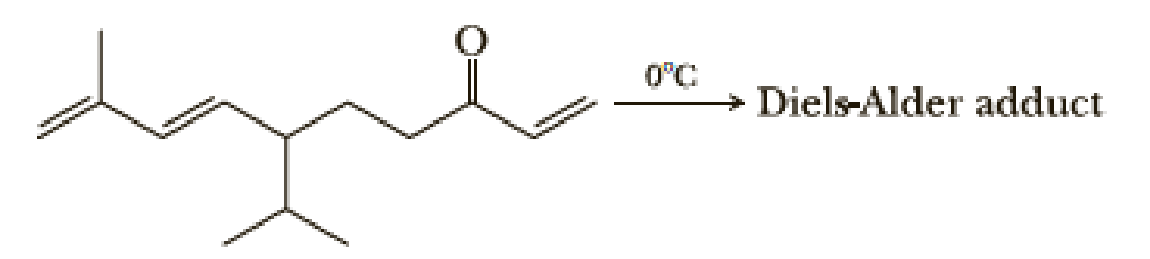
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Predict the major organic product(s) of the following reactions. Indicate which of the following mechanisms is in operation: SN1, SN2, E1, or E2.
(c)
(4pts)
Mechanism:
heat
(E1)
CH3OH
+
1.5pts each
_E1 _ (1pt)
Br
CH3OH
(d)
(4pts)
Mechanism:
SN1
(1pt)
(e)
(3pts)
1111 I
H
10
Ill!!
H
LDA
THF (solvent)
Mechanism: E2
(1pt)
NC
(f)
Bri!!!!!
CH3
NaCN
(3pts)
acetone
Mechanism: SN2
(1pt)
(SN1)
-OCH3
OCH3
1.5pts each
2pts for either product
1pt if incorrect
stereochemistry
H
Br
(g)
“,、
(3pts)
H
CH3OH
+21
Mechanism:
SN2
(1pt)
H
CH3
2pts
1pt if incorrect
stereochemistry
H
2pts
1pt if incorrect
stereochemistry
A mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixture
Chapter 20 Solutions
Organic Chemistry
Ch. 20.1 - Prob. 20.1PCh. 20.1 - Estimate the stabilization gained as a result of...Ch. 20.2 - Predict the product(s) formed by addition of one...Ch. 20.3 - Prob. 20.4PCh. 20.3 - Prob. 20.5PCh. 20.4 - Prob. 20.6PCh. 20.5 - Prob. 20.7PCh. 20.5 - Prob. 20.8PCh. 20.5 - Prob. 20.9PCh. 20.6 - Prob. 20.10P
Ch. 20.6 - Prob. 20.11PCh. 20.6 - Prob. 20.12PCh. 20 - If an electron is added to 1,3-butadiene, into...Ch. 20 - Prob. 20.15PCh. 20 - Predict the structure of the major product formed...Ch. 20 - Predict the major product formed by 1,4-addition...Ch. 20 - Predict the structure of the major 1,2-addition...Ch. 20 - Prob. 20.19PCh. 20 - Prob. 20.20PCh. 20 - Prob. 20.21PCh. 20 - Prob. 20.22PCh. 20 - Prob. 20.23PCh. 20 - Pyridine exhibits a UV transition of the type n at...Ch. 20 - Prob. 20.25PCh. 20 - Prob. 20.26PCh. 20 - Prob. 20.27PCh. 20 - Write the frontier molecular orbital analysis for...Ch. 20 - Prob. 20.29PCh. 20 - Draw structural formulas for the products of...Ch. 20 - Propose structural formulas for compounds A and B...Ch. 20 - Under certain conditions, 1,3-butadiene can...Ch. 20 - Prob. 20.33PCh. 20 - Prob. 20.34PCh. 20 - The following triene undergoes an intramolecular...Ch. 20 - Prob. 20.36PCh. 20 - Prob. 20.37PCh. 20 - Prob. 20.38PCh. 20 - Prob. 20.39PCh. 20 - The Diels-Alder reaction is not limited to making...Ch. 20 - The first step in a synthesis of dodecahedrane...Ch. 20 - Bicyclo-2,5-heptadiene can be prepared in two...Ch. 20 - Prob. 20.43PCh. 20 - Prob. 20.44PCh. 20 - Following is a retrosynthetic scheme for the...Ch. 20 - Prob. 20.46PCh. 20 - Prob. 20.47PCh. 20 - Prob. 20.48PCh. 20 - Prob. 20.49PCh. 20 - Prob. 20.50PCh. 20 - What reaction presented in this chapter is...Ch. 20 - Claisen rearrangement of an allyl phenyl ether...Ch. 20 - Prob. 20.53PCh. 20 - Prob. 20.54PCh. 20 - We now continue the use of organic chemistry...Ch. 20 - Write the products of the following sequences of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY