
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 20.6, Problem 20.10P
Interpretation Introduction
Interpretation: The synthesis of allyl phenyl ether and 2-butenyl phenyl ether from phenol and appropriate alkenyl halides have to be shown.
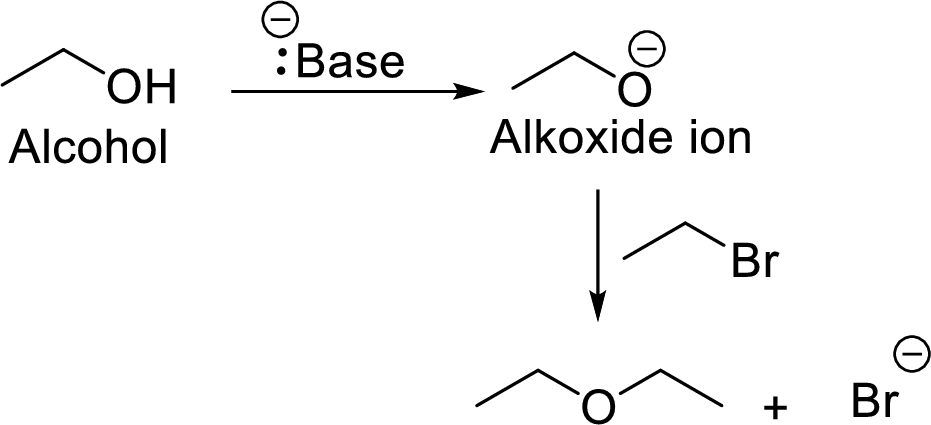
Concept Introduction:
It is the reaction in which an alkoxide or phenoxide ion reacts with an organohalide via
Example:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
These are in the wrong boxes. Why does the one on the left have a lower molar mass than the one on the right?
SYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants.
Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the
mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for
that step's mechanism.
CI
b.
a.
Use acetylene (ethyne)
and any alkyl halide as
your starting materials
Br
C.
d.
"OH
OH
III.
OH
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
Chapter 20 Solutions
Organic Chemistry
Ch. 20.1 - Prob. 20.1PCh. 20.1 - Estimate the stabilization gained as a result of...Ch. 20.2 - Predict the product(s) formed by addition of one...Ch. 20.3 - Prob. 20.4PCh. 20.3 - Prob. 20.5PCh. 20.4 - Prob. 20.6PCh. 20.5 - Prob. 20.7PCh. 20.5 - Prob. 20.8PCh. 20.5 - Prob. 20.9PCh. 20.6 - Prob. 20.10P
Ch. 20.6 - Prob. 20.11PCh. 20.6 - Prob. 20.12PCh. 20 - If an electron is added to 1,3-butadiene, into...Ch. 20 - Prob. 20.15PCh. 20 - Predict the structure of the major product formed...Ch. 20 - Predict the major product formed by 1,4-addition...Ch. 20 - Predict the structure of the major 1,2-addition...Ch. 20 - Prob. 20.19PCh. 20 - Prob. 20.20PCh. 20 - Prob. 20.21PCh. 20 - Prob. 20.22PCh. 20 - Prob. 20.23PCh. 20 - Pyridine exhibits a UV transition of the type n at...Ch. 20 - Prob. 20.25PCh. 20 - Prob. 20.26PCh. 20 - Prob. 20.27PCh. 20 - Write the frontier molecular orbital analysis for...Ch. 20 - Prob. 20.29PCh. 20 - Draw structural formulas for the products of...Ch. 20 - Propose structural formulas for compounds A and B...Ch. 20 - Under certain conditions, 1,3-butadiene can...Ch. 20 - Prob. 20.33PCh. 20 - Prob. 20.34PCh. 20 - The following triene undergoes an intramolecular...Ch. 20 - Prob. 20.36PCh. 20 - Prob. 20.37PCh. 20 - Prob. 20.38PCh. 20 - Prob. 20.39PCh. 20 - The Diels-Alder reaction is not limited to making...Ch. 20 - The first step in a synthesis of dodecahedrane...Ch. 20 - Bicyclo-2,5-heptadiene can be prepared in two...Ch. 20 - Prob. 20.43PCh. 20 - Prob. 20.44PCh. 20 - Following is a retrosynthetic scheme for the...Ch. 20 - Prob. 20.46PCh. 20 - Prob. 20.47PCh. 20 - Prob. 20.48PCh. 20 - Prob. 20.49PCh. 20 - Prob. 20.50PCh. 20 - What reaction presented in this chapter is...Ch. 20 - Claisen rearrangement of an allyl phenyl ether...Ch. 20 - Prob. 20.53PCh. 20 - Prob. 20.54PCh. 20 - We now continue the use of organic chemistry...Ch. 20 - Write the products of the following sequences of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning