
(a)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
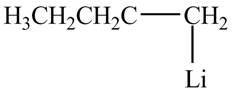
Figure 1
Explanation of Solution
The halogen group of
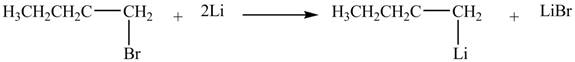
Figure 2
The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
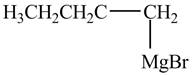
Figure 3
Explanation of Solution
The halogen group of alkyl halide forms bond with
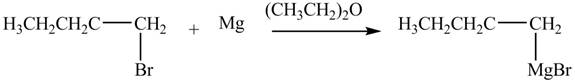
Figure 4
The product formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 5
Explanation of Solution
The halogen group of alkyl halide is replaced by
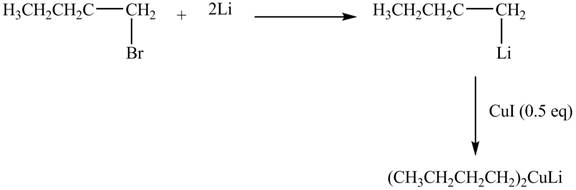
Figure 6
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 7
Explanation of Solution
The product formed by the reaction of
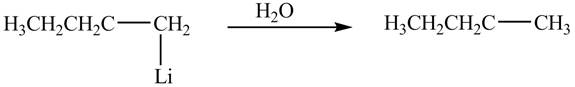
Figure 8
The product formed by the treatment of
(e)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 9
Explanation of Solution
The product formed by the reaction of
![]()
Figure 10
The product formed by the treatment of
(f)
Interpretation: The product formed by the treatment of
Concept introduction: The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of products with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 20.39P
The product formed by the treatment of
![]()
Figure 11
Explanation of Solution
The product formed by the reaction of
![]()
Figure 12
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry-Package(Custom)
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





