
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.11P
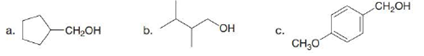
Draw the structure of both an acid chloride and an ester that can be used to prepare each compound by reduction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How to draw the reaction mechasnism below
Name the following molecules with IUpac
What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?
Chapter 20 Solutions
Organic Chemistry-Package(Custom)
Ch. 20 - Prob. 20.1PCh. 20 - Which carbonyl groups in the anticancer drug taxol...Ch. 20 - Prob. 20.3PCh. 20 - Prob. 20.4PCh. 20 - Problem 20.5 What aldehyde or ketone is needed to...Ch. 20 - Prob. 20.6PCh. 20 - Problem 20.7 Draw the products formed when is...Ch. 20 - Prob. 20.8PCh. 20 - Prob. 20.9PCh. 20 - Prob. 20.10P
Ch. 20 - Draw the structure of both an acid chloride and an...Ch. 20 - Problem 20.12 Draw the products formed from ...Ch. 20 - Prob. 20.13PCh. 20 - Prob. 20.14PCh. 20 - What product is formed when...Ch. 20 - Prob. 20.16PCh. 20 - Problem-20.16 Review the oxidation reactions using...Ch. 20 - Problem-20.17 Write the step(s) needed to convert ...Ch. 20 - Problem-20.18 Oct-1-yne reacts rapidly with ,...Ch. 20 - Draw the product formed when each organometallic...Ch. 20 - Draw the product of each reaction. a.c. b.d.Ch. 20 - Draw the products including stereochemistry of the...Ch. 20 - What Grignard reagent and carbonyl compound are...Ch. 20 - Problem 20.24 Linalool (the Chapter 9 opening...Ch. 20 - Problem 20.25 What Grignard reagent and carbonyl...Ch. 20 - Prob. 20.26PCh. 20 - Draw the products formed when each compound is...Ch. 20 - What ester and Grignard reagent are needed to...Ch. 20 - What organocuprate reagent is needed to convert...Ch. 20 - What reagent is needed to convert (CH3)2CHCH2CHO...Ch. 20 - Prob. 20.31PCh. 20 - What carboxylic acid formed from each alkyl halide...Ch. 20 - Prob. 20.33PCh. 20 - Prob. 20.34PCh. 20 - Problem 20.35 Synthesize each compound from...Ch. 20 - Prob. 20.36PCh. 20 - 20.37 Devise a synthesis of each alcohol from...Ch. 20 - 20.38 Draw the products formed when pentanal is...Ch. 20 - Prob. 20.39PCh. 20 - Draw the product formed when CH3CH2CH2MgBr is...Ch. 20 - Draw the product formed when (CH3CH2CH2CH2)2CuLi...Ch. 20 - The stereochemistry of the products of reduction...Ch. 20 - Prob. 20.43PCh. 20 - What reagent is needed to convert...Ch. 20 - What reagent is needed to convert...Ch. 20 - Draw the products or each reduction reaction. a....Ch. 20 - Prob. 20.47PCh. 20 - Draw all stereoisomers formed in each reaction. a....Ch. 20 - Prob. 20.49PCh. 20 - 20.46 Treatment of ketone A with ethynylithium...Ch. 20 - 20.47 Explain why metal hydride reduction gives an...Ch. 20 - Prob. 20.52PCh. 20 - 20.49 Identify the lettered compounds in the...Ch. 20 - Prob. 20.54PCh. 20 - Draw a stepwise mechanism for each reaction. a. b.Ch. 20 - Prob. 20.56PCh. 20 - 20.54 Draw a stepwise mechanism for the following...Ch. 20 - What Grignard reagent and aldehyde or ketone are...Ch. 20 - Prob. 20.59PCh. 20 - What ester and Grignard reagent are needed to...Ch. 20 - What organolithium reagent and carbonyl compound...Ch. 20 - 20.59 What epoxide and organometallic reagent are...Ch. 20 - Prob. 20.63PCh. 20 - 20.61 Propose two different methods to synthesize...Ch. 20 - Synthesize each compound from cyclohexanol using...Ch. 20 - Prob. 20.66PCh. 20 - Prob. 20.67PCh. 20 - Devise three different methods to prepare each...Ch. 20 - Convert benzene into each compound. You may also...Ch. 20 - Design a synthesis of each compound from alcohols...Ch. 20 - Synthesize each compound from the given starting...Ch. 20 - 20.69 An unknown compound A (molecular formula )...Ch. 20 - 20.70 Treatment of compound C (molecular formula )...Ch. 20 - 20.71 Treatment of compound E (molecular formula )...Ch. 20 - 20.72 Reaction of butanenitrile () with methyl...Ch. 20 - 20.73 Treatment of isobutene with forms a...Ch. 20 - Prob. 20.77PCh. 20 - Prob. 20.78PCh. 20 - 20.76 Lithium tri-sec-butylborohydride, also known...Ch. 20 - Prob. 20.80PCh. 20 - Prob. 20.81PCh. 20 - Prob. 20.82PCh. 20 - 20.80 Draw a stepwise mechanism for the following...Ch. 20 - Prob. 20.84P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License