
Concept explainers
What product is formed when
(a)
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Alkenes on hydrogenation reaction in the presence of
Answer to Problem 20.15P
The product formed by the treatment of
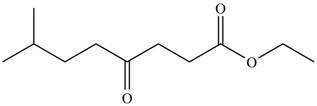
Figure 1
Explanation of Solution
The given compound
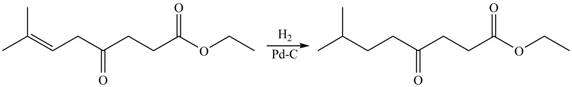
Figure 2
The product formed by the treatment of
(b)
Interpretation: The product formed by the treatment of
Concept introduction: Alkenes on hydrogenation reaction in the presence of
Answer to Problem 20.15P
The product formed by the treatment of
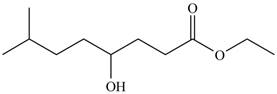
Figure 3
Explanation of Solution
The given compound
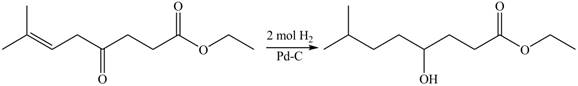
Figure 4
The product formed by the treatment of
(c)
Interpretation: The product formed by the treatment of
Concept introduction: Alkenes on hydrogenation reaction in the presence of
Answer to Problem 20.15P
The product formed by the treatment of
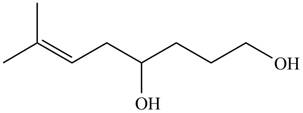
Figure 5
Explanation of Solution
The given compound
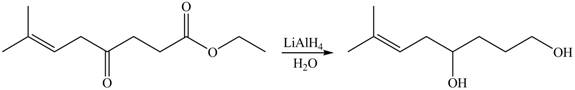
Figure 6
The product formed by the treatment of
(d)
Interpretation: The product formed by the treatment of
Concept introduction: Alkenes on hydrogenation reaction in the presence of
Answer to Problem 20.15P
The product formed by the treatment of
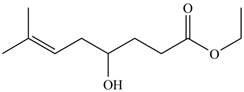
Figure 7
Explanation of Solution
The given compound
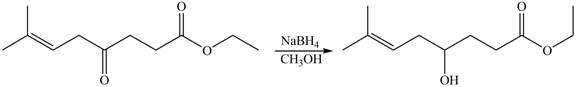
Figure 8
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry-Package(Custom)
- What is the lowest energy chair for the following cyclohexane? ' || || a. b. " " d.arrow_forwardAnswer the following questions using the below figure: Potential Energy ри Reaction Progress a. How many transition states occur in this reaction? b. How many intermediates occur in this reaction? c. Is this reaction spontaneous or nonspontaneous? d. Does this reaction have a positive or negative AG? e. Label the activation energy(ies).arrow_forwardDraw the following molecule as a chair in the lowest energy conformation. Then perform a chair flip. Brarrow_forward
- The U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m3 . If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 Larrow_forwardDuring a(n) ________ process, energy is transferred from the system to the surroundings. exothermic endothermic thermodynamic thermochemical physicalarrow_forwardUse the following information to determine the enthalpy for the reaction shown below. → S(s) + O2(g) SO2(9) ΔΗ Π ? Reference reactions: S(s) + O2(g) SO3(9) 2SO2(g) + O2(g) → 2SO3(g) AHxn = -395kJ AHrxn = ― -198kJarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





