
Concept explainers
(a)
Interpretation:
The systematic names of the given
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
2-methylbut-1-ene.
Explanation of Solution
The given structure is:
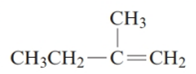
The parent chain in the given structure is of 4-carbon atoms so, prefix will be but. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 1 for double bond and the substituent on 2 position is methyl.
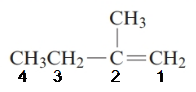
So, the IUPAC name will be: 2-methylbut-1-ene.
(b)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
2, 4-dimethylpent-1, 4-diene.
Explanation of Solution
The given structure is:
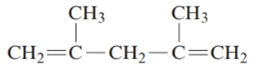
The parent chain in the given structure is of 5-carbon atoms so, prefix will be pent. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 1 and 4 for double bond and the substituent on 2 and 4 position is methyl.
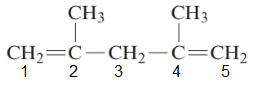
So, the IUPAC name will be: 2, 4-dimethylpent-1, 4-diene.
(c)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkene, the suffix “ane” of the corresponding alkane is replaced by “ene”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
6-ethyl-2-methyloct-4-ene.
Explanation of Solution
The given structure is:
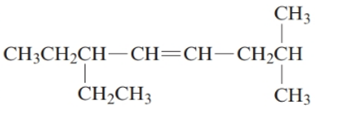
The parent chain in the given structure is of 5-carbon atoms so, prefix will be oct. Numbering is done in such a way that the multiple bond that is double bond gets lower number that is 4 for double bond and the substituent on 2 position is methyl and on 6 position is ethyl.
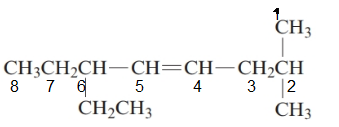
So, the IUPAC name will be: 6-ethyl-2-methyloct-4-ene.
(d)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
3-bromohept-1-yne.
Explanation of Solution
The given structure is:
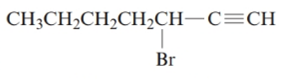
The parent chain in the given structure is of 7-carbon atoms so, prefix will be hept. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 1 for triple bond. The substituent on 3 position is bromo.
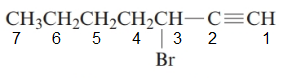
So, the IUPAC name will be: 3-bromohept-1-yne.
(e)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
7-chloro-2, 5, 5-trimethylhept-3-yne.
Explanation of Solution
The given structure is:
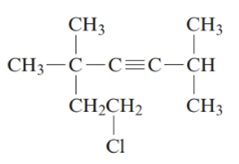
The parent chain in the given structure is of 7-carbon atoms so, prefix will be hept. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 3 for triple bond. The substituent on 2 and 5 position is methyl and on 7 position is chloro.
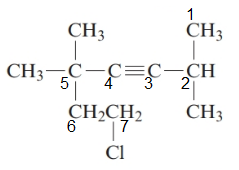
So, the IUPAC name will be: 7-chloro-2, 5, 5-trimethylhept-3-yne.
(f)
Interpretation:
The systematic names of the given unsaturated hydrocarbon should be determined.
Concept Introduction:
The hydrocarbon compounds that is compound containing only hydrogen and carbon atoms that contains multiple bond(s) are said to be unsaturated hydrocarbon. Compounds containing double bonds are said to be alkene whereas compounds containing triple bonds are said to be alkyne.
In order to give the name to the unsaturated hydrocarbon following steps are followed:
1. The parent (longest) continuous carbon chain containing multiple bonds between the carbon atoms is selected.
2. While writing the name of alkyne, the suffix “ane” of the corresponding alkane is replaced by “yne”.
3. Name should be written in alphabetical order and numbering should be done in such a way that the multiple bond and substituent group gets lowest number.
4. Hyphen is used to connect the number to the name.
For number of carbons atoms in alkane chain, the prefix is given as:
Carbon-1meth
Carbon-2eth
Carbon-3prop
Carbon-4but
Carbon-5pent
Carbon-6hex
Carbon-7hept
Carbon-8oct
Carbon-9non
Carbon-10dec.
Answer to Problem 144CP
4-ethyl-3-methyloct-1-yne.
Explanation of Solution
The given structure is:
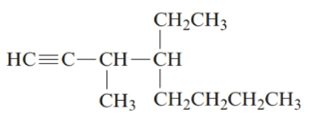
The parent chain in the given structure is of 8-carbon atoms so, prefix will be oct. Numbering is done in such a way that the multiple bond that is triple bond gets lower number that is 1 for triple bond. The substituent on 3 position is methyl and on 4 position is ethyl.
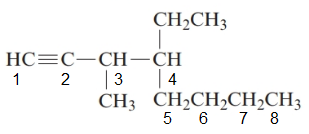
So, the IUPAC name will be: 4-ethyl-3-methyloct-1-yne.
Want to see more full solutions like this?
Chapter 20 Solutions
Introductory Chemistry: A Foundation
- 1,4-Dimethyl-1,3-cyclohexadiene can undergo 1,2- or 1,4-addition with hydrogen halides. (a) 1,2-Addition i. Draw the carbocation intermediate(s) formed during the 1,2-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,2-addition product formed during the reaction in (i)? (b) 1,4-Addition i. Draw the carbocation intermediate(s) formed during the 1,4-addition of hydrobromic acid to 1,4-dimethyl-1,3-cyclohexadiene. ii. What is the major 1,4-addition product formed from the reaction in (i)? (c) What is the kinetic product from the reaction of one mole of hydrobromic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (d) What is the thermodynamic product from the reaction of one mole of hydrobro-mic acid with 1,4-dimethyl-1,3-cyclohexadiene? Explain your reasoning. (e) What major product will result when 1,4-dimethyl-1,3-cyclohexadiene is treated with one mole of hydrobromic acid at - 78 deg * C ? Explain your reasoning.arrow_forwardGive the product of the bimolecular elimination from each of the isomeric halogenated compounds. Reaction A Reaction B. КОВ CH₂ HotBu +B+ ко HOIBU +Br+ Templates More QQQ Select Cv Templates More Cras QQQ One of these compounds undergoes elimination 50x faster than the other. Which one and why? Reaction A because the conformation needed for elimination places the phenyl groups and to each other Reaction A because the conformation needed for elimination places the phenyl groups gauche to each other. ◇ Reaction B because the conformation needed for elimination places the phenyl groups gach to each other. Reaction B because the conformation needed for elimination places the phenyl groups anti to each other.arrow_forwardFive isomeric alkenes. A through each undergo catalytic hydrogenation to give 2-methylpentane The IR spectra of these five alkenes have the key absorptions (in cm Compound Compound A –912. (§), 994 (5), 1643 (%), 3077 (1) Compound B 833 (3), 1667 (W), 3050 (weak shoulder on C-Habsorption) Compound C Compound D) –714 (5), 1665 (w), 3010 (m) 885 (3), 1650 (m), 3086 (m) 967 (5), no aharption 1600 to 1700, 3040 (m) Compound K Match each compound to the data presented. Compound A Compound B Compound C Compound D Compoundarrow_forward
- 7. The three sets of replicate results below were accumulated for the analysis of the same sample. Pool these data to obtain the most efficient estimate of the mean analyte content and the standard deviation. Lead content/ppm: Set 1 Set 2 Set 3 1. 9.76 9.87 9.85 2. 9.42 9.64 9.91 3. 9.53 9.71 9.42 9.81 9.49arrow_forwardDraw the Zaitsev product famed when 2,3-dimethylpentan-3-of undergoes an El dehydration. CH₂ E1 OH H₁PO₁ Select Draw Templates More QQQ +H₂Oarrow_forwardComplete the clean-pushing mechanism for the given ether synthesia from propanol in concentrated sulfurica140°C by adding any mining aloms, bands, charges, nonbonding electron pairs, and curved arrows. Draw hydrogen bonded to cayan, when applicable. ore 11,0 HPC Step 1: Draw curved arrows Step 2: Complete the intend carved Q2Q 56 QQQ Step 3: Complete the intermediate and add curved Step 4: Modify the structures to draw the QQQ QQQarrow_forward
- 6. In an experiment the following replicate set of volume measurements (cm3) was recorded: (25.35, 25.80, 25.28, 25.50, 25.45, 25.43) A. Calculate the mean of the raw data. B. Using the rejection quotient (Q-test) reject any questionable results. C. Recalculate the mean and compare it with the value obtained in 2(a).arrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. • If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T G OH де OH This transformation can't be done in one step.arrow_forwardMacmillan Leaming Draw the major organic product of the reaction. 1. CH3CH2MgBr 2. H+ - G Select Draw Templates More H о QQarrow_forward
- Draw the condensed structure of 3-hydroxy-2-butanone. Click anywhere to draw the first atom of your structure.arrow_forwardGive the expected major product of reaction of 2,2-dimethylcyclopropane with each of the following reagents. 2. Reaction with dilute H₂SO, in methanol. Select Draw Templates More CHC Erase QQQ c. Reaction with dilute aqueous HBr. Select Drew Templates More Era c QQQ b. Reaction with NaOCH, in methanol. Select Draw Templates More d. Reaction with concentrated HBr. Select Draw Templates More En a QQQ e. Reaction with CH, Mg1, then H*, H₂O 1. Reaction with CH,Li, then H', H₂Oarrow_forwardWrite the systematic name of each organic molecule: structure O OH OH name X ☐arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





