
General Chemistry: Principles and Modern Applications (11th Edition)
11th Edition
ISBN: 9780132931281
Author: Ralph H. Petrucci, F. Geoffrey Herring, Jeffry D. Madura, Carey Bissonnette
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 11E
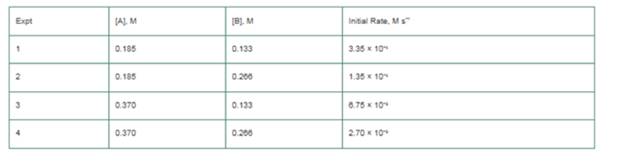
The initial
a. What is the order of reaction with respect to A and to B?
b. What is the overall reaction order?
c. What is the value of the rate constant, k?
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule05:23
Students have asked these similar questions
In the solid state, oxalic acid occurs as
a dihydrate with the formula H2C2O4
C+2H2O. Use this formula to
calculate the formula weight of oxalic
acid. Use the calculated formula
weight and the number of moles
(0.00504mol)
of oxalic acid in each titrated
unknown sample recorded in Table
6.4 to calculate the number of grams
of pure oxalic acid dihydrate
contained in each titrated unknown
sample.
1.
Consider a pair of elements with 2p and 4p valence orbitals (e.g., N and Se). Draw their
(2p and 4p AO's) radial probability plots, and sketch their angular profiles. Then, consider these
orbitals from the two atoms forming a homonuclear л-bond. Which element would have a
stronger bond, and why?
(4 points)
Write the reaction and show the mechanism of the reaction. Include the mechanism
for formation of the NO2+
2. Explain, using resonance structures, why the meta isomer is formed. Draw possible
resonance structures for ortho, meta and para.
Chapter 20 Solutions
General Chemistry: Principles and Modern Applications (11th Edition)
Ch. 20 - In the reaction 2A+BC+3D , reactant A is found to...Ch. 20 - From Figure 20-2 estimate the rate of reaction at...Ch. 20 - In the reaction A products, [A] is found to be...Ch. 20 - In the reaction A products, at t = 0. [A]= 0.1565...Ch. 20 - In the reaction A products. 4.40 min after the...Ch. 20 - Refer to Experiment 2 of Table 20.3 and to...Ch. 20 - For the reaction A+2BC ,the rate of reaction is...Ch. 20 - If the rate of reaction (20.3) is 5.7104 M s-1 ,...Ch. 20 - In the reaction A(g)B(g)+C(g) , the totalpressure...Ch. 20 - At 65C , the half-life for the first-order...
Ch. 20 - The initial rate of the reaction A+BC+D is...Ch. 20 - For the reaction A+BC+D , the following initial...Ch. 20 - Prob. 13ECh. 20 - The following data are obtained for the initial...Ch. 20 - One of the following statements is true and the...Ch. 20 - One of the following statements true and the other...Ch. 20 - The first-order reaction A products has t1/2=180...Ch. 20 - The reaction A products is first order in A....Ch. 20 - The reaction A products is first order A. a. If...Ch. 20 - In the first-order reaction A products, [A] =...Ch. 20 - In the first-order reaction A products, it found...Ch. 20 - The half-life of me radioactive isotope...Ch. 20 - Acetoacetic acid, CH2COOH2COOH , a reagent in...Ch. 20 - The following first-order reaction occurs in...Ch. 20 - For the reaction A- products, the following data...Ch. 20 - The decomposition of dimethyl ether at 504C is (...Ch. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - Prob. 29ECh. 20 - Prob. 30ECh. 20 - Prob. 31ECh. 20 - Prob. 32ECh. 20 - [Hint: There are several of arrivivg at answer for...Ch. 20 - [Hint: There are several ways of arrivivig at...Ch. 20 - Prob. 35ECh. 20 - Prob. 36ECh. 20 - For the reaction A products, the following data...Ch. 20 - Prob. 38ECh. 20 - For the reaction A products, the data tabulated...Ch. 20 - For the reaction A2B+C , the following data are...Ch. 20 - In three different experiments, the following...Ch. 20 - Ammonia decomposes on the surface of a hot...Ch. 20 - Prob. 43ECh. 20 - Consider three hypothetical reactions A — products...Ch. 20 - Prob. 45ECh. 20 - If even tiny sped is introduced into a mixture of...Ch. 20 - For me reversible reaction A+BC+D , the enthalpy...Ch. 20 - Prob. 48ECh. 20 - By inspection of the reaction profile for the...Ch. 20 - By inspection of the reaction profile for the...Ch. 20 - The rate constant for the reaction...Ch. 20 - At what temperature will the rate constant for the...Ch. 20 - Prob. 53ECh. 20 - The reaction C2H5+OHC2H5OH+I was studied in an...Ch. 20 - The first-order reaction A products has a...Ch. 20 - For the first-order reaction N2O4(g)2NO2+12O2g...Ch. 20 - Prob. 57ECh. 20 - Concerning the rule of thumb stated r Exercise 57,...Ch. 20 - The following statements about catalysis are not...Ch. 20 - Prob. 60ECh. 20 - What are the similarities and differences between...Ch. 20 - Certain gas-phase reactions on a heterogeneous...Ch. 20 - The graph show s the effect of enzyme...Ch. 20 - The graph shows the effect of temperature on...Ch. 20 - Prob. 65ECh. 20 - Prob. 66ECh. 20 - The reaction 2NO+2H2N2+2H2O is second order m [NO]...Ch. 20 - The mechanism proposed for me reaction of H2(g)...Ch. 20 - The reaction 2NO+Cl22NOCl has rate law: rate of...Ch. 20 - A simplified rate law 1o the reaction 2O2(g)3O2(g)...Ch. 20 - Prob. 71ECh. 20 - One proposed meachanism for the condensation of...Ch. 20 - Suppose that the reaction r Example 20-8 is first...Ch. 20 - [A]t as a function of time for the reaction A —...Ch. 20 - Exactly 300 s after decomposition of H2O2(aq)...Ch. 20 - Use the method of Exercise 75 to determine the...Ch. 20 - Prob. 77IAECh. 20 - Prob. 78IAECh. 20 - Hydroxide ion is involved in the mechanism of the...Ch. 20 - The half-life for the first-order decomposition of...Ch. 20 - The decomposition of ethylene oxide at 690 K is...Ch. 20 - Prob. 82IAECh. 20 - The following data are for the reaction 2 A + B ...Ch. 20 - Prob. 84IAECh. 20 - Prob. 85IAECh. 20 - Prob. 86IAECh. 20 - The following three-step mechanism has been...Ch. 20 - Prob. 88IAECh. 20 - Prob. 89IAECh. 20 - Prob. 90IAECh. 20 - Prob. 91IAECh. 20 - Prob. 92IAECh. 20 - Prob. 93IAECh. 20 - You want to test the following proposed mechanism...Ch. 20 - Prob. 95IAECh. 20 - Benzenediazonium chloride decomposes by a...Ch. 20 - The object is to study the kinetics of the...Ch. 20 - Prob. 98SAECh. 20 - Prob. 99SAECh. 20 - Explain the important distinctions between each...Ch. 20 - Prob. 101SAECh. 20 - A first-order reaction A — products, has a...Ch. 20 - Prob. 103SAECh. 20 - Prob. 104SAECh. 20 - The rate of a chemical reaction generally...Ch. 20 - For the reaction A+B2C, which proceeds by a...Ch. 20 - Prob. 107SAECh. 20 - Prob. 108SAECh. 20 - Prob. 109SAECh. 20 - For me reaction A products the following data are...Ch. 20 - For the reaction A+2BC+D , the rate law is rate...Ch. 20 - Prob. 112SAECh. 20 - If the plot of the reactant concentration versus...Ch. 20 - Prob. 114SAECh. 20 - Prob. 115SAE
Additional Science Textbook Solutions
Find more solutions based on key concepts
18. SCIENTIFIC THINKING By measuring the fossil remains of Homo floresiensis, scientists have estimated its wei...
Campbell Biology: Concepts & Connections (9th Edition)
If a compound has a molecular ion with an odd-numbered mass, then the compound contains an odd number of nitrog...
Organic Chemistry (8th Edition)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
33. Consider the unbalanced chemical equation.
A chemistry student tries to balance the equation by placing th...
Introductory Chemistry (6th Edition)
10.71 Identify each of the following as an acid or a base: (10.1)
H2SO4
RbOH
Ca(OH)2
HI
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Q1. Which wavelength of light has the highest frequency?
a) 10 nm
b) 10 mm
c) 1 nm
d) 1 mm
Chemistry: A Molecular Approach (4th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forward3. A molecular form of "dicarbon", C2, can be generated in gas phase. Its bond dissociation energy has been determined at 599 kJ/mol. Use molecular orbital theory to explain why energy of dissociation for C₂+ is 513 kJ/mol, and that for C2² is 818 kJ/mol. (10 points)arrow_forward9.73 g of lead(IV) chloride contains enough Cl- ions to make ____ g of magnesium chloride.arrow_forward
- 6. a) C2's. Phosphorus pentafluoride PF5 belongs to D3h symmetry group. Draw the structure of the molecule, identify principal axis of rotation and perpendicular (4 points) b) assume that the principal axis of rotation is aligned with z axis, assign symmetry labels (such as a1, b2, etc.) to the following atomic orbitals of the P atom. (character table for this group is included in the Supplemental material). 3s 3pz (6 points) 3dz²arrow_forward2. Construct Lewis-dot structures, and draw VESPR models for the ions listed below. a) SiF5 (4 points) b) IOF4 (4 points)arrow_forward5. Complex anion [AuCl2]¯ belongs to Doh symmetry point group. What is the shape of this ion? (4 points)arrow_forward
- 4. Assign the following molecules to proper point groups: Pyridine N 1,3,5-triazine N Narrow_forward7. a) Under normal conditions (room temperature & atmospheric pressure) potassium assumes bcc lattice. Atomic radius for 12-coordinate K atom is listed as 235 pm. What is the radius of potassium atom under normal conditions? (3 points) b) Titanium metal crystallyzes in hcp lattice. Under proper conditions nitrogen can be absorbed into the lattice of titanium resulting in an alloy of stoichiometry TiNo.2. Is this compound likely to be a substitutional or an interstitial alloy? (Radius of Ti (12-coordinate) is 147 pm; radius of N atom is 75 pm. (3 points)arrow_forwardcan someone answer the questions and draw out the complete mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY