
ORGANIC CHEMISTRY (LL)-PACKAGE
8th Edition
ISBN: 9781319316389
Author: VOLLHARDT
Publisher: MAC HIGHER
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 34P
Interpretation Introduction
Interpretation: The acid-base reactions with curved arrows should be identified.
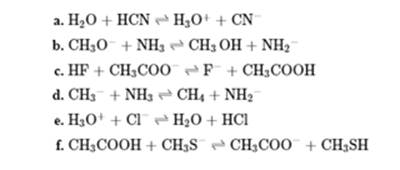
Concept introduction: In a typical acid-base reaction the fundamental principle is a lone pair of base reaches out for an acidic proton. Similar curved arrows are used to show the movement of electrons.. After deprotonation, the species left with a negative charge is referred to as the conjugate base of acid while the other with a positive charge is termed conjugate acid of given base. For example;
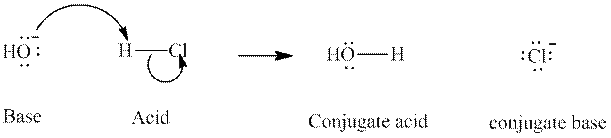
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has a weak conjugate base and the strong base has weak conjugate acid and vice-versa.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Select the stronger base from each pair of compounds.
(a) H₂CNH₂ or EtzN
(b)
CI
or
NH2
NH2
(c)
.Q
or EtzN
(d)
or
(e)
N
or
(f)
H
or
H
4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a.
2.
1. LDA
3. H3O+
HO
b.
H3C CH3
H3O+
✓ H
OH
Chapter 2 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning