
Concept explainers
(a)
Interpretation:
True and false
Meaning of “energy is quantized” that only certain energy values are allowed.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on it own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
Electrons might be promoted only to the higher energy orbitals of certain fixed energy values; the value in between are not allowed. Thus, the given statement is True.
(b)
Interpretation:
True and false
According to Bohr energy of an electron in an atom is quantized.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The electron in an atom don’t move freely in the space around the nucleus. The electrons move in certain fixed orbitals which have certain energy levels. Thus, the energy of the electrons in an atom is quantized. Therefore, the provided statement is True.
(c)
Interpretation:
True and false
Electrons present in the atoms are confined to regions of space known as “principle energy levels”.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
Though there is significantly large space outside the nucleus, the electrons are confined to particular regions around the nucleus. These regions are called the “principle energy levels” or shells. Therefore, the provided statement is True.
(d)
Interpretation:
True and false
Each principal energy level might hold the maximum of two electrons.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
Each principal energy level or shell contains of varying number of subshells s, p, d, f. Thus, the number of electrons in each principal energy level also varies. The subshells (s) might hold a maximum of the two electrons, while the subshells p, d, f might hold the maximum 8, 18 and 32 electrons respectively. Therefore, the provided statement is False.
(e)
Interpretation:
True and false
An electron in a 1s orbital is held closer to the nucleus than an electron in a 2s orbitals.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The 1s orbital lies in the first principal energy level, whereas the 2s orbital lies in the second principal energy level. The initial principal energy level is nearer to the nucleus as compared to the second. Therefore, the electron in the 1s orbital is nearer to the nucleus than that in the 2s orbital. Therefore, the provided statement is True.
(f)
Interpretation:
True and false
An electron in a 2s orbital is harder to remove from an atom than an electron in a 2s orbital.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
The electron in the 1s orbital is closer to the nucleus than the electron in the 2s orbital. So, the nuclear attraction on the electrons in the 1s orbital is greater than on those in the 2s orbital. Therefore, a higher energy is needed to remove the inner 1s electron compared to the 2s electron. So, the electron in 1s orbital is harder to remove from an atom than an electron in a 2s orbital. Therefore, the provided statement is False.
(g)
Interpretation:
True and false
An s orbital has the shape of a sphere, with the nucleus at the center of the sphere.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The shapes of the orbitals represent the electron density that is the probability of finding the electrons. For an s orbital, the electron density is spherical around the nucleus. Therefore, the provided statement is True.
(h)
Interpretation:
True and false
Each 2p orbital has the shape of a dumbbell, with the nucleus at the midpoint of the dumbbell.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
For a 2p orbital the electron density is a dumbbell shaped, with the nucleus at the midpoint of the dumbbell. Therefore, the provided statement is True.
(i)
Interpretation:
True and false
The three 2p orbitals in an atom are aligned parallel to each other.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
Each 2p orbital has the shape of the dumbbell, and the three 2p orbitals 2px, 2py, 2pz are at the right angles to each other with each orbital on x, y, z axis. Therefore, the provided statement is False.
(j)
Interpretation:
True and false
An orbital is a region of space that can hold two electrons.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
In an atom, shells are divided into subshells, and within these subshells, electrons are grouped in orbitals with each orbital holding a maximum of two electrons. Therefore, the provided statement is True.
(k)
Interpretation:
True and false
The second shell contains one ‘s’ orbital and three ‘p’ orbitals.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The second shell can hold a maximum of eight electrons. These electrons can occupy the 2s and 2p orbitals. The 2s orbital is a single s orbital and holds two electrons. The 2p orbitals in sets of three and hold six electrons. Thus, the second shell one s orbital and three p orbitals. Therefore, the provided statement is True.
(l)
Interpretation:
True and false
In the ground-state electron configuration of an atom, only the lowest-energy orbitals are occupied.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The electron configuration of an atom provides description of the orbitals in which the electrons are occupied. In the ground-state electron configuration, electrons occupy the orbital the orbital of lower energy first. All other orbitals of higher energy are empty. Therefore, the provided statement is True.
(m)
Interpretation:
True and false
A spinning electron behaves as a tiny bar magnet, with a North Pole and South Pole.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
A spinning electron produces a tiny magnetic field, aligning itself in the north-south direction. Thus, a spinning electron is considered as a tiny bar magnet, with a North Pole and a South Pole.
Therefore, the provided statement is True.
(n)
Interpretation:
True and false
An orbital can hold a maximum of two electrons with their spins paired.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
An orbital can hold a maximum of two electrons. When magnetic field of two electrons are aligned in opposite directions, the electrons are said to be spin-paired.
Therefore, the provided statement is True.
(o)
Interpretation:
True and false
Paired electrons spins mean that the two electrons are aligned with their spins North Pole to North Pole and South Pole to South Pole.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
When magnetic fields of two electrons are aligned in opposite directions, the electrons are said to be spin-paired. Paired electron spins mean that the two electrons are aligned with their spins, North Pole to South Pole and South Pole to North Pole.
Therefore, the provided statement is False.
(p)
Interpretation:
True and false
An orbital box diagram puts all of the electrons of an atom in one box with their spins aligned.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
The orbital box diagrams are used to represent the electrons. In this diagram, each box represents an orbital, so each box will hold a maximum of two electrons. An unpaired electron is represented by an arrow with its head up, whereas two electrons with paired spins are represented by a pair of arrows with heads in opposite directions. So the orbital box diagram doesn’t fill all of the electrons an atom in one box with their spins aligned.
Therefore, the provided statement is False.
(q)
Interpretation:
True and false
An orbital box diagram of a carbon atom shows two unpaired electrons.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
A neutral carbon atom has six electrons. Two electrons are placed in the 1s orbital and two electrons are placed in the 2s orbital. The electrons in 1s an 2s orbitals are paired. The remaining two electrons are placed each in 2px, 2py orbitals.
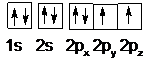
Therefore, the provided statement is True.
(r)
Interpretation:
True and false
A Lewis dot structure shows only the electrons in the valence shell of an atom of the element.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
When writing a Lewis dot structure for an atom, the
Therefore, the provided statement is True.
(s)
Interpretation:
True and false
A characteristic of Group 1A elements is that each has one unpaired electron in its outermost occupied (valence) shell.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
True.
Explanation of Solution
The group number provided the number of valence electrons in the outer shell of an atom. As the elements in Group 1A have only one valance electrons, it is always unpaired.
Therefore, the provided statement is True.
(t)
Interpretation:
True and false
A characteristic of Group 6A elements is that each has six unpaired electrons in its outermost occupied (valence) shell.
Concept Introduction:
There are four quantum numbers which explains the position of electrons in an atom. Below are details:
- Principle Quantum Number (n) - It explains the size of electron exist in the orbital.
- Angular Momentum Quantum Number (l) − It explains the orbitals’ shape.
- Magnetic Quantum Number (ml) − It explains about the orbital’s orientation in the space.
- Electron Spin Number (ms) − It explains about the direction which an electron spins on its own axis.
Answer to Problem 2.49P
False.
Explanation of Solution
The group number gives the number of valence electrons in the outer shell of an atom, and not the number of unpaired electrons. Group 6A elements have six valence electrons. Out of the six valence electrons, two electrons occupy the 2s orbital. The remaining four electrons occupy the 2p orbital such that two electrons are paired in a 2px orbital, whereas two unpaired electrons remain in 2py and 2pz.
Therefore, the provided statement is False.
Want to see more full solutions like this?
Chapter 2 Solutions
Introduction to General, Organic and Biochemistry
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





