
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
4th Edition
ISBN: 9781260562620
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19.5, Problem 19.9P
Review Section 5.2 on balancing chemical equations. Then, write a balanced equation for the complete combustion (or 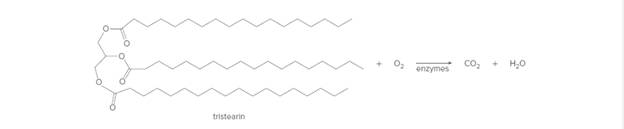
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used Ai solution
The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.
Electronic contribution to the heat capacity at constant volume
A) is always zero
B) is zero, except for excited levels whose energy is comparable to KT
C) equals 3/2 Nk
D) equals Nk exp(BE)
Chapter 19 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
Ch. 19.1 - Prob. 19.1PPCh. 19.1 - Prob. 19.1PCh. 19.2 - (a) Draw a skeletal structure for each fatty acid....Ch. 19.2 - Prob. 19.2PCh. 19.2 - Prob. 19.3PCh. 19.3 - Prob. 19.3PPCh. 19.3 - One component of jojoba oil is a wax formed from...Ch. 19.3 - Explain why beeswax is insoluble in water,...Ch. 19.3 - Prob. 19.6PCh. 19.4 - Prob. 19.4PP
Ch. 19.4 - Prob. 19.7PCh. 19.4 - Prob. 19.8PCh. 19.5 - Prob. 19.5PPCh. 19.5 - Review Section 5.2 on balancing chemical...Ch. 19.5 - Prob. 19.10PCh. 19.6 - Prob. 19.6PPCh. 19.6 - Identify the components of each lipid and classify...Ch. 19.7 - Prob. 19.12PCh. 19.7 - Prob. 19.13PCh. 19.7 - Prob. 19.14PCh. 19.8 - (a) Label the rings of the steroid nucleus in...Ch. 19.8 - Prob. 19.16PCh. 19.8 - Prob. 19.17PCh. 19.9 - Prob. 19.18PCh. 19.9 - Prob. 19.19PCh. 19.10 - Prob. 19.20PCh. 19.11 - Prob. 19.21PCh. 19.11 - Prob. 19.22PCh. 19 - Prob. 23PCh. 19 - Prob. 24PCh. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - Prob. 28PCh. 19 - Rank the fatty acids in order of increasing...Ch. 19 - Prob. 30PCh. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - Prob. 33PCh. 19 - Prob. 34PCh. 19 - Draw the structure of a wax formed from palmitic...Ch. 19 - Draw the structure of a wax formed from a...Ch. 19 - What hydrolysis products are formed when each wax...Ch. 19 - What hydrolysis products are formed when each wax...Ch. 19 - Prob. 39PCh. 19 - Prob. 40PCh. 19 - Draw a triacylglycerol that fits each description:...Ch. 19 - Draw a triacylglycerol that fits each description:...Ch. 19 - Draw the structure of a triacylglycerol that...Ch. 19 - Draw the structure of a triacylglycerol that...Ch. 19 - Consider the following four types of compounds:...Ch. 19 - How do fats and oils compare with respect to each...Ch. 19 - For the food product shown in the accompanying...Ch. 19 - For the food product shown in the accompanying...Ch. 19 - Answer the following questions about the given...Ch. 19 - Answer the following questions about the given...Ch. 19 - Draw the products formed when each triacylglycerol...Ch. 19 - Draw the products formed when each triacylglycerol...Ch. 19 - Which of the following are phospholipids: (a)...Ch. 19 - Prob. 54PCh. 19 - Prob. 55PCh. 19 - Prob. 56PCh. 19 - wIn transporting molecules or ions across a cell...Ch. 19 - Prob. 58PCh. 19 - Draw the structure of the anabolic steroid...Ch. 19 - Draw the structure of the anabolic steroid...Ch. 19 - Why must cholesterol be transported through the...Ch. 19 - Describe the role of HDLs and LDL5 in cholesterol...Ch. 19 - Prob. 63PCh. 19 - Prob. 64PCh. 19 - (a) Draw the structure of an estrogen and an...Ch. 19 - (a) Draw the structure of an androgen and a...Ch. 19 - What are the similarities and differences between...Ch. 19 - Why aren’t prostaglandins classified as hormones?Ch. 19 - What two structural features characterize all...Ch. 19 - List three biological functions of prostaglandins...Ch. 19 - Explain why aspirin and celecoxib differ in how...Ch. 19 - How does zileuton treat the cause of asthma, not...Ch. 19 - Answer each question with regard to vitamins A and...Ch. 19 - Answer each question in Problem 19.73 for vitamins...Ch. 19 - Give an example of each type of lipid. a. a...Ch. 19 - Give an example of each type of lipid. a. a...Ch. 19 - Consider each of the following components: [1]...Ch. 19 - Consider each of the following components: [1]...Ch. 19 - Block diagrams representing the general structures...Ch. 19 - For each block diagram in Problem 19.79, label the...Ch. 19 - Prob. 81PCh. 19 - Prob. 82PCh. 19 - Prob. 83PCh. 19 - Prob. 84PCh. 19 - Prob. 85PCh. 19 - Prob. 86PCh. 19 - Can an individual survive on a completely fat-free...Ch. 19 - Prob. 88PCh. 19 - Prob. 89PCh. 19 - Prob. 90PCh. 19 - Prob. 91PCh. 19 - Prob. 92PCh. 19 - Prob. 93CPCh. 19 - Prob. 94CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward
- CaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forwardIn the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forward
- In natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forwardMost ceramic materials have low thermal conductivities because:(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is very restricted by secondary bonds.arrow_forwardResistivity and electrical conductivity.(a) In metals, resistivity decreases.(b) In metals, resistivity decreases and conductivity in semiconductors also decreases with increasing temperature.(c) With increasing temperature, resistivity in metals and conductivity in semiconductors also increases.(d) None of the above.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY