
Concept explainers
(a)
Interpretation: The correct name should be given for the incorrectly named organic compound 2,4,6-tribromobenzene.
Concept Introduction:
Nomenclature of benzene and its derivative compounds:
Monosubstituted alkylbenzenes are named as derivatives of benzene; for example butylbenzene.
When one of the special case compounds (example aniline, toluene, phenol) is involved, the compound is named as a derivative of the special compound.
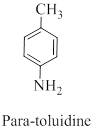
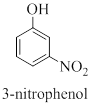
Nomenclature of benzene compound with more substituents,
If one of the substituents imparts a special name, name the molecule as a derivative of that parent.
If none of the substituents imparts a special name, number the substituents to give the smallest set of numbers, and list them in alphabetical order before the ending “benzene”.
Example:
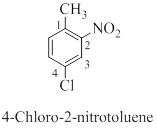
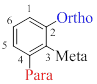
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)
(b)
Interpretation: The correct name should be given for the incorrectly named organic compound 3-hydroxynitrobenzene.
Concept introduction:
Nomenclature of benzene and its derivative compounds:
Monosubstituted alkylbenzenes are named as derivatives of benzene; for example butylbenzene.
When one of the special case compounds (example aniline, toluene, phenol…) is involved, the compound is named as a derivative of the special compound.
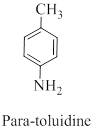

Nomenclature of benzene compound with more substituents,
If one of the substituents imparts a special name, name the molecule as a derivative of that parent.
If none of the substituents imparts a special name, number the substituents to give the smallest set of numbers, and list them in alphabetical order before the ending “benzene”.
Example:
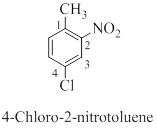
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

(c)
Interpretation: The correct name should be given for the incorrectly named organic compound
Concept introduction:
Nomenclature of benzene and its derivative compounds:
Monosubstituted alkylbenzenes are named as derivatives of benzene; for example butylbenzene.
When one of the special case compounds (example aniline, toluene, phenol…) is involved, the compound is named as a derivative of the special compound.
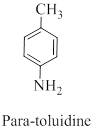

Nomenclature of benzene compound with more substituents,
If one of the substituents imparts a special name, name the molecule as a derivative of that parent.
If none of the substituents imparts a special name, number the substituents to give the smallest set of numbers, and list them in alphabetical order before the ending “benzene”.
Example:
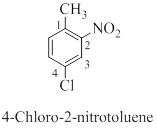
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

(d)
Interpretation: The correct name should be given for the incorrectly named organic compound 1,6-dichlorobenzene.
Concept introduction:
Nomenclature of benzene and its derivative compounds:
Monosubstituted alkylbenzenes are named as derivatives of benzene; for example butylbenzene.
When one of the special case compounds (example aniline, toluene, phenol…) is involved, the compound is named as a derivative of the special compound.
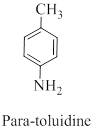

Nomenclature of benzene compound with more substituents,
If one of the substituents imparts a special name, name the molecule as a derivative of that parent.
If none of the substituents imparts a special name, number the substituents to give the smallest set of numbers, and list them in alphabetical order before the ending “benzene”.
Example:
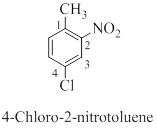
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- What is the stepwise mechanism for this reaction?arrow_forward32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forward
- K for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forwardDraw the principal organic product of the following reaction.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided structures, draw the curved arrows that epict the mechanistic steps for the proton transfer between a hydronium ion and a pi bond. Draw any missing organic structures in the empty boxes. Be sure to account for all lone-pairs and charges as well as bond-breaking and bond-making steps. 2 56°F Mostly cloudy F1 Drawing Arrows > Q Search F2 F3 F4 ▷11 H. H : CI: H + Undo Reset Done DELLarrow_forward
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbons. Draw out the benzene ring structure when doing itarrow_forward1) Calculate the longest and shortest wavelengths in the Lyman and Paschen series. 2) Calculate the ionization energy of He* and L2+ ions in their ground states. 3) Calculate the kinetic energy of the electron emitted upon irradiation of a H-atom in ground state by a 50-nm radiation.arrow_forwardCalculate the ionization energy of He+ and Li²+ ions in their ground states. Thannnxxxxx sirrr Ahehehehehejh27278283-4;*; shebehebbw $+$;$-;$-28283773838 hahhehdvaarrow_forward
- Plleeaasseee solllveeee question 3 andd thankss sirr, don't solve it by AI plleeaasseee don't use AIarrow_forwardCalculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





