
Concept explainers
(a)
Interpretation: The structure of the given compound para-toluidine has to be drawn.
Concept introduction:
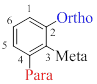
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)
Aryl
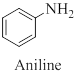
Toluidine is an aryl amine whose structure is similar to aniline except that a methyl group is substituted onto the benzene ring.
(b)
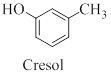
Interpretation: The structure of the given compound meta-cresol has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Phenol is an
Cresol is a derivative of phenol which contains a methyl group other than the
(c)
Interpretation: The structure of the given compound para-xylene has to be drawn.
Concept Introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Dimethyl benzene compounds are known as xylene. Depends on the position of methyl group it can be exist as ortho, para and Meta compound.
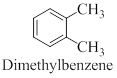
(d)
Interpretation:
The structure of the given compound ortho-chlorobenzenesulfonic acid has to be drawn.
Concept introduction:
To indicate relative position on a benzene ring, three terms are mainly used and they are,
- (1) Ortho-(o): On adjacent carbons (1,2)
- (2) Meta-(m): Separated by one carbon (1,3)
- (3) Para-(p): Separated by two carbons (1,4)

Benzene sulfonic acid is an organosulfur compound with the following structure.
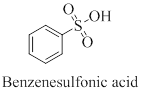
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
- 8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forwardChoose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward
- 7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forwardWhat is the skeletal structure of the product of the following organic reaction?arrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardPlease help me answer the following questions using the data I included. 1&2arrow_forwardAssign all the Protons in HNMRarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





