
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
7th Edition
ISBN: 9780134240152
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 92P
What reagents are required to carry out the following synthesis?
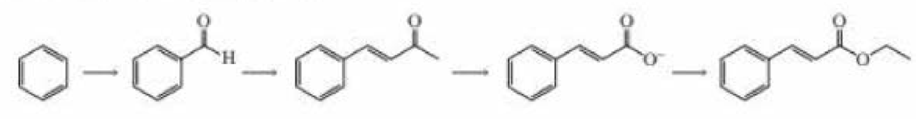
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.
Please help me solve number 2
Choose the best reagents to complete the following reaction.
오
Na2Cr2O7
H2SO4, H2O
Problem 22 of 35
A
Na2Cr2O7
H2SO4, H2O
H2/Pt
B
pressure
OH
1. NaBH4
C
2. H3O+
D
DMP (Dess-Martin Periodinane)
CH2Cl2
CrO3
Done
Dramabana_Minor
Submit
Chapter 19 Solutions
Organic Chemistry; Modified MasteringChemistry with Pearson eText -- ValuePack Access Card; Study Guide and Student Solutions Manual for Organic Chemistry, Books a la Carte Edition (7th Edition)
Ch. 19.1 - Draw the structure for each of the following: a....Ch. 19.2 - PROBLEM 2
If electrophilic addition to benzene is...Ch. 19.4 - Why does hydration inactivate FeBr3?Ch. 19.6 - Prob. 5PCh. 19.7 - Prob. 6PCh. 19.8 - What is the major product of a Friedel-Crafts...Ch. 19.10 - Describe two ways to prepare each of the following...Ch. 19.12 - Prob. 9PCh. 19.13 - Name the following:Ch. 19.13 - Prob. 12P
Ch. 19.13 - Prob. 13PCh. 19.13 - Prob. 14PCh. 19.14 - Prob. 15PCh. 19.14 - List the compounds in each set from most reactive...Ch. 19.15 - Prob. 18PCh. 19.15 - What product(s) result from nitration of each of...Ch. 19.15 - Prob. 20PCh. 19.16 - Which acid in each of the following pairs is...Ch. 19.16 - Prob. 23PCh. 19.16 - Prob. 24PCh. 19.18 - Show how the following compounds can be...Ch. 19.18 - Give the products, if any, of each of the...Ch. 19.19 - a. Does a coupling reaction have to be used to...Ch. 19.19 - PROBLEM 28
Show how each of the following...Ch. 19.20 - What is the major product(s) of each of the...Ch. 19.20 - Prob. 30PCh. 19.21 - Why isn't FeBr3 used as a catalyst in the first...Ch. 19.21 - Prob. 33PCh. 19.21 - Write the sequence of steps required for the...Ch. 19.21 - Prob. 35PCh. 19.22 - What product is formed from reaction of...Ch. 19.22 - Prob. 37PCh. 19.22 - Draw the structure of the activated ring and the...Ch. 19.23 - Prob. 39PCh. 19.23 - Prob. 40PCh. 19.23 - Diazomethane can be used to convert a carboxylic...Ch. 19.24 - Prob. 42PCh. 19.24 - Prob. 43PCh. 19.24 - Prob. 44PCh. 19.25 - Prob. 45PCh. 19 - Draw the structure for each of the following: a....Ch. 19 - Prob. 47PCh. 19 - Prob. 48PCh. 19 - Prob. 49PCh. 19 - For each of the statements in Column I, choose a...Ch. 19 - What product is obtained from the reaction of...Ch. 19 - Draw the product(s) of each of the following...Ch. 19 - Rank the following substituted anilines from most...Ch. 19 - Prob. 54PCh. 19 - The compound with the 1H NMR spectrum shown below...Ch. 19 - Prob. 56PCh. 19 - Show how the following compounds can be...Ch. 19 - Prob. 58PCh. 19 - Rank each group of compounds from most reactive to...Ch. 19 - Prob. 60PCh. 19 - Describe two ways to prepare anisole from benzene.Ch. 19 - For each of the following components, indicate the...Ch. 19 - Prob. 63PCh. 19 - Prob. 64PCh. 19 - Prob. 65PCh. 19 - Prob. 66PCh. 19 - An aromatic hydrocarbon with a molecular formula...Ch. 19 - The following tertiary alkyl bromides undergo an...Ch. 19 - Show how the following compounds can be...Ch. 19 - Use the four compounds shown below to answer the...Ch. 19 - a. Rank the following esters from most reactive to...Ch. 19 - A mixture of 0.10 mol benzene and 0.10 mol...Ch. 19 - Prob. 73PCh. 19 - Benzene underwent a Friedel-Crafts acylation...Ch. 19 - Prob. 75PCh. 19 - Prob. 76PCh. 19 - Prob. 77PCh. 19 - Friedel-Crafts alkylations can be carried out with...Ch. 19 - Show how the following compounds can be prepared...Ch. 19 - Prob. 80PCh. 19 - Prob. 81PCh. 19 - a. Describe four ways the following reaction can...Ch. 19 - Propose a mechanism for each of the following...Ch. 19 - Prob. 84PCh. 19 - Describe how naphthalene can he prepared from the...Ch. 19 - Prob. 86PCh. 19 - Using resonance contributors for the carbocation...Ch. 19 - Prob. 88PCh. 19 - When heated with chromic acid, compound A forms...Ch. 19 - Prob. 90PCh. 19 - Prob. 91PCh. 19 - What reagents are required to carry out the...Ch. 19 - Show how the following compounds can be prepared...Ch. 19 - An unknown compound reacts with ethyl chloride and...Ch. 19 - How can you distinguish the following compounds...Ch. 19 - P-Fluoronitrobenzene is more reactive toward...Ch. 19 - a. Explain why the following reaction leads to the...Ch. 19 - Describe how mescaline can be synthesized from...Ch. 19 - Propose a mechanism for the following reaction...Ch. 19 - Explain why hydroxide ion catalyzes the reaction...Ch. 19 - Propose a mechanism for each of the following...Ch. 19 - Prob. 102PCh. 19 - Prob. 103PCh. 19 - Describe how 3-methyl-1-phenyl-3-pentanol can he...Ch. 19 - a. How can aspirin be synthesized from benzene? b....Ch. 19 - Prob. 106PCh. 19 - Show how Novocain, a painkiller used frequently by...Ch. 19 - Prob. 108PCh. 19 - Saccharin, an artificial sweetener, is about 300...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-cyclopentyl-2-methyl-1,3-dioxolane with H3O+. Draw the structures of the compounds.arrow_forward
- Question 4 For the molecule shown below, (7 marks): A) Sketch the Newman projection for the view looking along the bond from the perspective of the arrow. B) Then, draw the Newman projection for each 60° rotation along the bond until it returns to the starting point. C) Clearly indicate which Newman projection is the one we see in the structure shown below, and clearly indicate which Newman projection is the highest in energy and which is the lowest in energy. H H Me 'H Me Mearrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the amine side product. 'N' 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forwardSubmit Problem 3 of 10 Draw the major product of this reaction. Ignore inorganic byproducts and the amine side product. O 'N' NH 1. NaOH, heat 2. Neutralizing work-up Select to Drawarrow_forward
- b) Certain cyclic compounds are known to be conformationally similar to carbohydrates, although they are not themselves carbohydrates. One example is Compound C shown below, which could be imagined as adopting four possible conformations. In reality, however, only one of these is particularly stable. Circle the conformation you expect to be the most stable, and provide an explanation to justify your choice. For your explanation to be both convincing and correct, it must contain not only words, but also "cartoon" orbital drawings contrasting the four structures. Compound C Possible conformations (circle one): Детarrow_forwardLab Data The distance entered is out of the expected range. Check your calculations and conversion factors. Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3? Did you report your data to the correct number of significant figures? - X Experimental Set-up HCI-NH3 NH3-HCI Longer Tube Time elapsed (min) 5 (exact) 5 (exact) Distance between cotton balls (cm) 24.30 24.40 Distance to cloud (cm) 9.70 14.16 Distance traveled by HCI (cm) 9.70 9.80 Distance traveled by NH3 (cm) 14.60 14.50 Diffusion rate of HCI (cm/hr) 116 118 Diffusion rate of NH3 (cm/hr) 175.2 175.2 How to measure distance and calculate ratearrow_forwardFor the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically: 1:1 (one mole of EDTA per mole of metal ion) 2:1 (two moles of EDTA per mole of metal ion) 1:2 (one mole of EDTA per two moles of metal ion) None of the abovearrow_forward
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY