
Concept explainers
(a)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate
Glycolysis cycle. In this cycle, the glucose is converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons
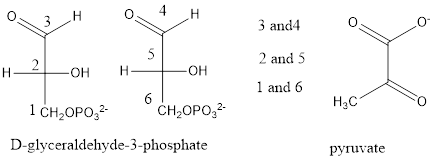
(b)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate metabolism, the conversion of glucose into pyruvate is known as
Glycolysis cycle. In this cycle the glucose converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons atoms numbered in the order of where it is in glucose,
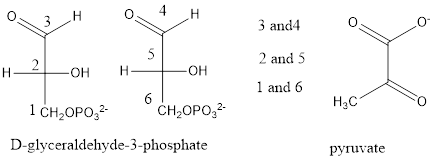
(c)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate metabolism, the conversion of glucose into pyruvate is known as
Glycolysis cycle. In this cycle the glucose converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons atoms numbered in the order of where it is in glucose,
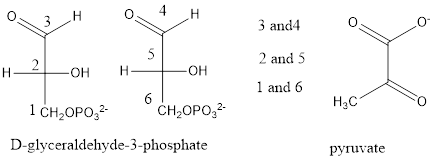
(d)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate metabolism, the conversion of glucose into pyruvate is known as
Glycolysis cycle. In this cycle the glucose converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons atoms numbered in the order of where it is in glucose,
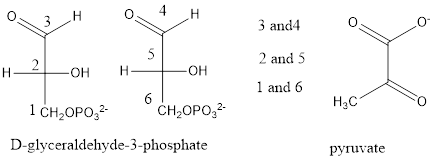
(e)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate metabolism, the conversion of glucose into pyruvate is known as
Glycolysis cycle. In this cycle the glucose converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons atoms numbered in the order of where it is in glucose,
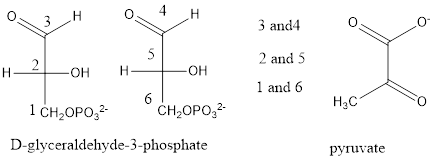
(f)
Interpretation:
The labeled carbon in pyruvate should be determined when it is comes from
Glycolysis cycle:
In the carbohydrate metabolism, the conversion of glucose into pyruvate is known as
Glycolysis cycle. In this cycle the glucose converted into D-glyceraldehye-3-phosphate and this D-glyceraldehye-3-phosphate is further converted into pyruvate,
In this process, the carbons atoms numbered in the order of where it is in glucose,
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- H3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forward
- Is the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forward
- If CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward+ Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning





