
(a)
Interpretation:
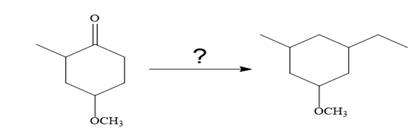
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Alkylation of carbonyl compound can take place at the α-carbon via nucleophilic substitution reaction. In this reaction acidic proton of the carbonyl compound is abstracted by the strong base an enolate anion is formed. The attack of this nucleophile takes place at the electrophilic carbon of the alkyl bromide and bromine atom is displaced through the
Answer to Problem 19.73P
The given synthesis is carried out as:
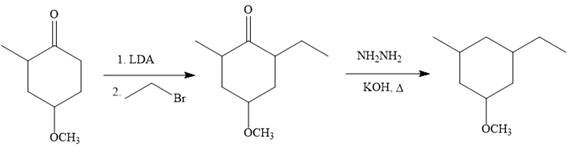
Explanation of Solution
The given synthesis:
So the given synthesis is carried out below:
In the first step, the nucleophilic addition of the enolate anion to the ethyl bromide takes place shown below:
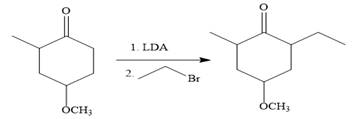
Note than LDA is sterically hindered strong base, therefore, abstract less sterically hindered proton selectively.
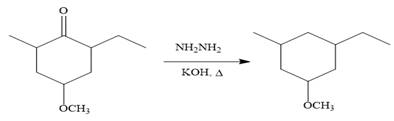
Finally, the carbonyl group is reduced to a corresponding methylene group (
It is shown how to carry out the given synthesis by using any reagents necessary.
(b)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Alkylation of carbonyl compound can take place at the α carbon via nucleophilic substitution reaction. In this reaction acidic proton of the carbonyl compound is abstracted by the strong base an enolate anion is formed. The attack of this nucleophile takes place at the electrophilic carbon of the alkyl bromide and bromine atom is displaced through
Answer to Problem 19.73P
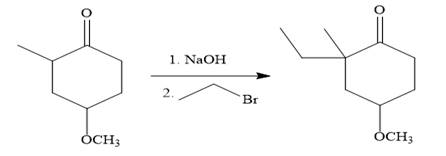
The given synthesis is carried out as:
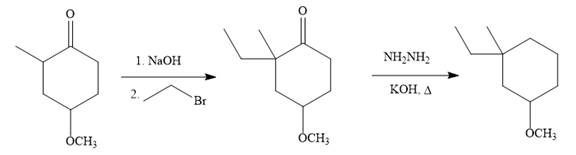
Explanation of Solution
The given synthesis is
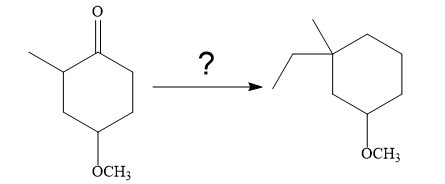
So the given synthesis is carried out below:
In the first step, the nucleophilic addition of the enolate anion to the ethyl bromide takes place shown below:
The require nucleophile is generated by the treatment of the strong base
Finally, the carbonyl group is reduced to a corresponding methylene group (
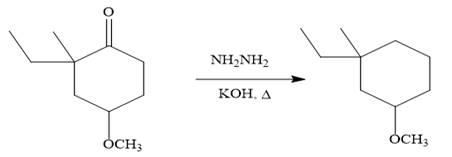
It is shown how to carry out the given synthesis by using any reagents necessary.
(c)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
Lithium dialkylcuprate,
Answer to Problem 19.73P
The given synthesis is carried out as:
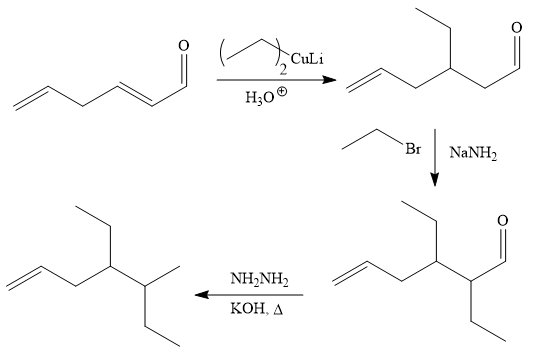
Explanation of Solution
The given synthesis is
It is noticed that one ethyl group is added to the 3-position of the original compound, therefore, the conjugate addition must be needed.
So the given synthesis is carried out below:
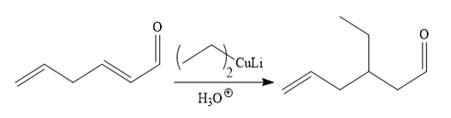
The first conjugate addition is carried out by the treatment of lithium diethylcuprate, a Gilman reagent with the given starting material. Since the conjugate addition is irreversible to the carbonyl group and regenerated during the workup step.
The above product then treated with ethyl bromide in the presence of a strong base
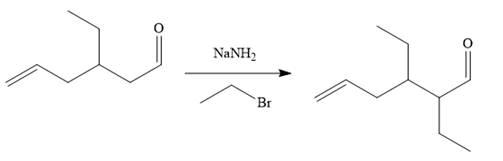
Finally, the carbonyl group is reduced to a corresponding methyl group (
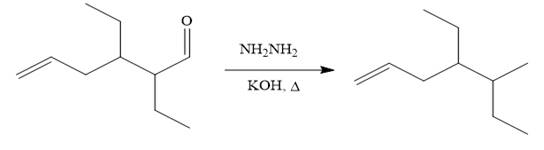
It is shown how to carry out the given synthesis by using any reagents necessary.
(d)
Interpretation:
It is to be shown how to carry out the given synthesis by using any reagents necessary.
Concept introduction:
LDA is the strong non-nucleophilic base, due to bulky isopropyl groups. Therefore, it abstract proton of less sterically hindered α-carbon of the carbonyl compound. The reduction of the carbonyl group of a ketone to a methylene (
Answer to Problem 19.73P
The given synthesis is carried out as:
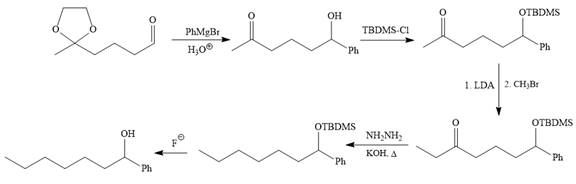
Explanation of Solution
The given synthesis is
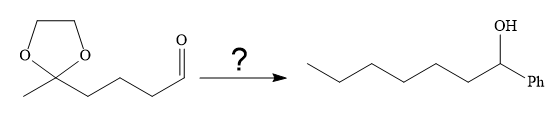
It is noticed that the phenyl group is added to the carbonyl carbon of the given starting material. So Grignard reaction carried out first as below:
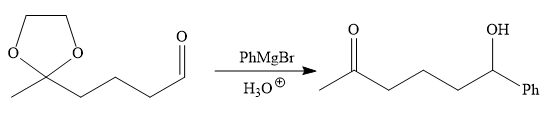
Since acidic condition required in workup step of Grignard reaction, the protected carbonyl group deprotected automatically because in acidic condition acetal protection also deprotect and the carbonyl group regenerated (right to the above product).
The alkylation of right carbon of the regenerated carbonyl compound is needed and it is done by using the sterically hindered base, LDA followed by methyl bromide. The bromine atom is replaced by a nucleophile (an enolate anion) via
The substrate is first treated with
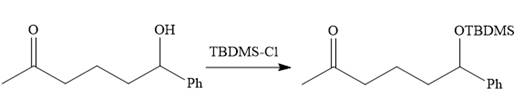
Now the required alkylation is carried out as below:
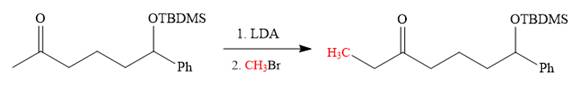
Finally, the carbonyl group is reduced to a corresponding methylene group (
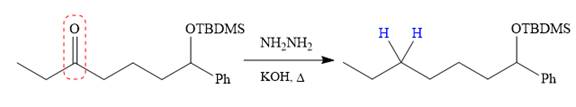
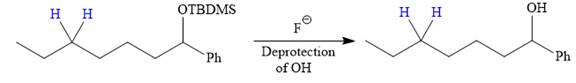
The protected OH in the form of OTBDMS is deprotected by the use of the source of
It is shown how to carry out the given synthesis by using any reagents necessary.
Want to see more full solutions like this?
Chapter 19 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Part III. Arrange the following carbons (in blue) in order of increasing chemical shift. HO B NH 2 A CIarrow_forward6. Choose the compound that will produce the spectrum below and assign the signals as carbonyl, aryl, or alkyl. 100 ō (ppm) 50 0 7. 200 150 Assign all of the protons on the spectrum below. 8. A B 4 E C 3 ō (ppm) 2 1 0 Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. OH 6 OH 3 2 1 0 4 ō (ppm)arrow_forwardIn the Thermo Fisher application note about wine analysis (Lesson 3), the following chromatogram was collected of nine components of wine. If peak 3 has a retention time of 3.15 minutes and a peak width of 0.070 minutes, and peak 4 has a retention time of 3.24 minutes and a peak width of 0.075 minutes, what is the resolution factor between the two peaks? [Hint: it will help to review Lesson 2 for this question.] MAU 300 200 T 34 5 100- 1 2 CO 6 7 8 9 0 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0 4.2 4.4 4.6 4.8 5.0 5.2 Minutes 3.22 0.62 1.04 O 1.24arrow_forward
- The diagram shows two metals, A and B, which melt at 1000°C and 1400°C. State the weight percentage of the primary constituent (grains of C) that would be obtained by solidifying a 20% alloy of B. 1000°C a+L L+C 900°С 12 α a+C 45 1200 C L+y 140096 C+Y a+ß 800°C 700°C C+B 96 92 a+B 0 10 20 30 40 50 60 70 80 90 100 A % peso B Barrow_forward8. Choose the compound that will produce the spectrum below and assign the signals to the corresponding protons. 2 4 3 ō (ppm) OH 4 6 6 СОН 2 1 0arrow_forward7. Assign all of the protons on the spectrum below. A B 2 C E 2 1 3 6 4 3 2 1 0arrow_forward
- e. If (3R,4R)-3,4-dichloro-2,5-dimethylhexane and (3R,4S)-3,4-dichloro-2,5-dimethylhexane are in a solution at the same concentration, would this solution be expected to rotate plane polarized light (that is, be optically active)? Please provide your reasoning for your answer. [If you read this problem carefully, you will not need to draw out the structures to arrive at your answer...]arrow_forward1. How many neighbors does the proton that produces the multiplet below have? 2. 3. اللـ Draw a partial structure from the multiplet below. (The integration of the multiplet is 6) M Using the additivity constants found in appendix G of your lab manual, calculate the approximate chemical shifts of the protons indicated below. (Show your work!!!) B A Br SHarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve this long problem. Thanksarrow_forward
- 4. An 'H-NMR of a compound is acquired. The integration for signal A is 5692 and the integration for signal B is 25614. What is the simplest whole number ratio of protons for signals A and B? (Show your work!!!) 5. Assign the carbons in the NMR below as either carbonyl, aromatic, or alkyl. 200 150 100 50 ō (ppm) 1arrow_forwardSpeaking of composite materials, indicate the correct option:(A). Composite materials can only be: metal-polymer or polymer-polymer.(B). Composite materials can be made up of particles, but not fibers or sheets.(C). When the reinforcing particles are uniformly distributed in a composite material, there may be a greater tendency for it to have isotropic properties.(D). None of the above is correct.arrow_forwardIf we are talking about viscoelastic modulus or viscoelastic relaxation modulus in polymers, indicate the correct option.(A). It reports the variation of elastic behavior as a function of time.(B). It is only useful for defining its glass transition temperature.(C). It only allows us to define the polymer degradation temperature.(D). Neither option is correct.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





