
Concept explainers
(a)
Interpretation:
The
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is
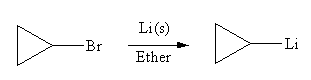
Explanation of Solution
The given reaction is,
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:
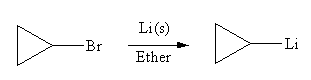
The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(b)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF).
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
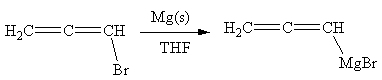
Explanation of Solution
The given reaction is
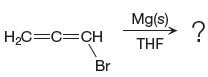
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF). So the C-Br bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(c)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF).
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
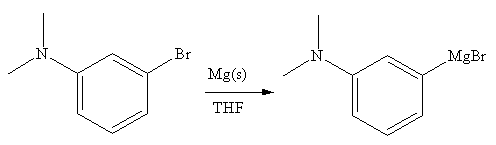
Explanation of Solution
The given reaction is
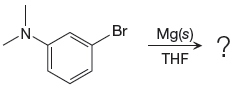
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl bromide can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether solvent such as tetrahydrofuran (THF). So the C-Br bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(d)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyl bromide can be converted to a Grignard reagent (RMgX) simply by treating it with solid magnesium in an ether solvent.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
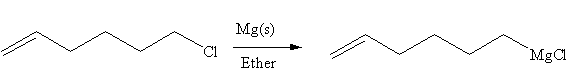
Explanation of Solution
The given reaction is
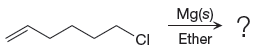
In the above reaction, the C atom of the C-Cl bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyl chloride can be converted to a Grignard reagent (RMgBr) simply by treating it with solid magnesium in an ether. So the C-Cl bond will become C-Mg bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:
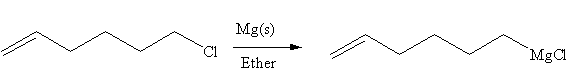
The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(e)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
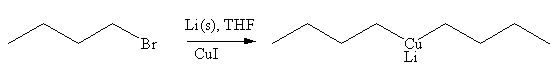
Explanation of Solution
The given reaction is
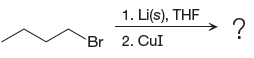
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. Lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI. So the C-Br bond will become C-CuLi bond. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(f)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether solvent such as THF.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
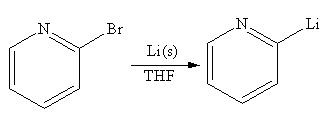
Explanation of Solution
The given reaction is
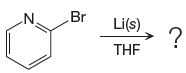
In the above reaction, the C atom of the C-Br bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. An alkyllithium reagent (RLi) can be synthesized from an alkyl bromide by treating it with solid lithium in ether solvent such as THF. So the C-Br bond will become C-Li bond. Therefore the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
(g)
Interpretation:
The organometallic compound that would be produced by the given reaction is to be drawn.
Concept introduction:
To form a bond between two carbons of like charge, a separate reaction that first reverses the charge (or polarity) at one of the carbons is carried out. Thus, one carbon atom would become electron rich while the other would remain electron poor. This general idea of reversing a charge at a particular atom is called umpolung. In the alkyl halide reactant, the C atom bonded to the halogen atom bears a partial positive charge and is relatively electron poor. By contrast, that C atom has to become electron rich in the organometallic compound produced. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI.
Answer to Problem 19.41P
The organometallic compound that would be produced by the given reaction is shown below:
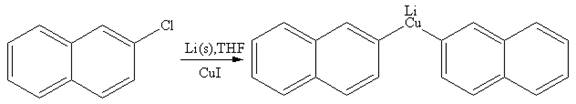
Explanation of Solution
The given reaction is
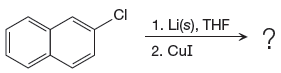
In the above reaction, the C atom of the C-Cl bond is electron-poor. When the C-Br bond is bonded to the metal, the carbon will become electron-rich. A lithium dialkylcuprate is synthesized from the corresponding alkyllithium reagent by treating it with copper(I) iodide, CuI. Therefore, the organometallic compound that would be produced by the given reaction is as shown below:

The organometallic compound that would be produced by the given reaction is drawn by converting the electron-poor carbon of C-Br bond to an electron-rich carbon.
Want to see more full solutions like this?
Chapter 19 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
- Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward
- 5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forwardplease help fill in the tablearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



