
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357119303
Author: Bettelheim, Frederick A., Brown, William H., Campbell, Mary K., FARRELL, Shawn O., Torres, Omar
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 19, Problem 16P
Interpretation Introduction
Interpretation:
The conventions for using a and ß to designate the configurations of the cyclic forms of monosaccharides.
Concept Introduction:
The cyclic forms of monosaccharides exist in two forms a and ß depending on the configuration at C-1 carbon atom.
The structures for alpha and beta form of glucose are as follows:
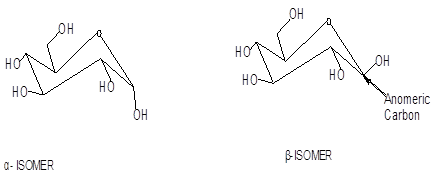
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Give detailed mechanism Solution with explanation needed. Don't give Ai generated solution
Show work with explanation needed....don't give Ai generated solution
1.
6. Draw the products for the following reaction:
2.
Diels-Aider
reaction
NOH O
OH
Chapter 19 Solutions
Introduction to General, Organic and Biochemistry
Ch. 19.1 - Prob. 19.1QCCh. 19.1 - Prob. 19.2QCCh. 19.2 - Prob. 19.3QCCh. 19.3 - Prob. 19.4QCCh. 19.4 - Prob. 19.5QCCh. 19.5 - Prob. 19.6QCCh. 19 - Prob. 1PCh. 19 - Prob. 2PCh. 19 - Prob. 3PCh. 19 - Prob. 4P
Ch. 19 - Prob. 5PCh. 19 - Prob. 6PCh. 19 - Prob. 7PCh. 19 - Prob. 8PCh. 19 - Prob. 9PCh. 19 - Prob. 10PCh. 19 - Prob. 11PCh. 19 - Prob. 12PCh. 19 - Prob. 13PCh. 19 - Prob. 14PCh. 19 - Prob. 15PCh. 19 - Prob. 16PCh. 19 - Prob. 17PCh. 19 - Prob. 18PCh. 19 - Prob. 19PCh. 19 - Prob. 20PCh. 19 - Prob. 21PCh. 19 - Prob. 22PCh. 19 - Prob. 23PCh. 19 - Prob. 24PCh. 19 - Prob. 25PCh. 19 - Prob. 26PCh. 19 - Prob. 27PCh. 19 - Prob. 28PCh. 19 - Prob. 29PCh. 19 - Prob. 30PCh. 19 - Prob. 31PCh. 19 - Prob. 32PCh. 19 - Prob. 33PCh. 19 - Prob. 34PCh. 19 - Prob. 35PCh. 19 - Prob. 36PCh. 19 - Prob. 37PCh. 19 - Prob. 38PCh. 19 - Prob. 39PCh. 19 - Prob. 40PCh. 19 - Prob. 41PCh. 19 - 6 Where is glycogen stored in the human body?Ch. 19 - Prob. 43PCh. 19 - 8 How is it possible that cows can digest grass...Ch. 19 - 1 Hyaluronic acid acts as a lubricant in the...Ch. 19 - Prob. 46PCh. 19 - Prob. 47PCh. 19 - Prob. 48PCh. 19 - Prob. 49PCh. 19 - Prob. 50PCh. 19 - Prob. 51PCh. 19 - Prob. 52PCh. 19 - Prob. 53PCh. 19 - Prob. 54PCh. 19 - Prob. 55PCh. 19 - Prob. 56PCh. 19 - Prob. 57PCh. 19 - Prob. 58PCh. 19 - Prob. 59PCh. 19 - Prob. 60PCh. 19 - Prob. 61PCh. 19 - Prob. 62PCh. 19 - Prob. 63PCh. 19 - Prob. 64PCh. 19 - Prob. 65PCh. 19 - Prob. 66PCh. 19 - Prob. 67PCh. 19 - Prob. 68PCh. 19 - Prob. 69PCh. 19 - Prob. 70PCh. 19 - Prob. 71PCh. 19 - Prob. 72PCh. 19 - Prob. 73PCh. 19 - Prob. 74PCh. 19 - Prob. 75P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning