
Fundamentals of Physics Extended
10th Edition
ISBN: 9781118230725
Author: David Halliday, Robert Resnick, Jearl Walker
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 45P
SSM ILW A gas within a closed chamber undergoes the cycle shown in the p-V diagram of Fig. 18-39. The horizontal scale is set by Vs = 4.0 m3. Calculate the net energy added to the system as heat during one complete cycle.
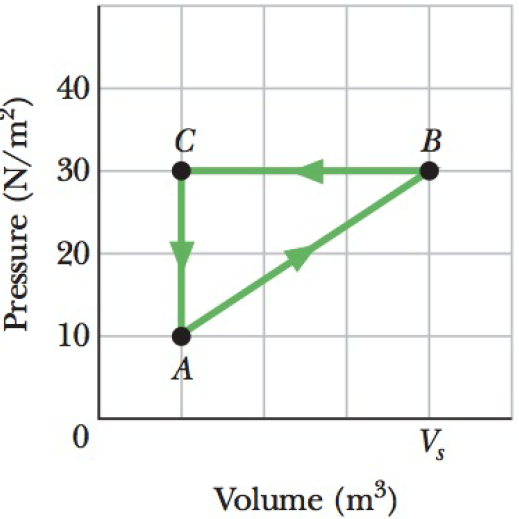
Figure 18-39 Problem 45.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If a 1/2 inch diameter drill bit spins at 3000 rotations per minute, how fast is the outer edge moving as it contacts a piece of metal while drilling a machine part?
Need help with the third question (C)A gymnast weighing 68 kg attempts a handstand using only one arm. He plants his hand at an angl reesulting in the reaction force shown.
Q: What is the direction of the force on the current carrying conductor in the
magnetic field in each of the cases 1 to 8 shown below?
(1)
B
B
B into page
X X X
x
X X X X
(2)
B
11 -10°
B
x I
B
I out of page
(3)
I into page
(4)
B out of page
out of page
I
N
N
S
x X X X
I
X
X X X
I
(5)
(6)
(7)
(8)
S
Chapter 18 Solutions
Fundamentals of Physics Extended
Ch. 18 - The initial length L, change in temperature T, and...Ch. 18 - Figure 18-24 shows three linear temperature...Ch. 18 - Materials A, B, and C are solids that are at their...Ch. 18 - A sample A of liquid water and a sample B of ice,...Ch. 18 - Question 4 continued: Graphs b through f of Fig....Ch. 18 - Figure 18-26 shows three different arrangements of...Ch. 18 - Figure 18-27 shows two closed cycles on p-V...Ch. 18 - For which cycle in Fig. 18-27, traversed...Ch. 18 - Three different materials of identical mass are...Ch. 18 - A solid cube of edge length r, a solid sphere of...
Ch. 18 - A hot object is dropped into a thermally insulated...Ch. 18 - Suppose the temperature of a gas is 373.15 K when...Ch. 18 - Two constant-volume gas thermometers are...Ch. 18 - A gas thermometer is constructed of two...Ch. 18 - a In 1964, the temperature in the Siberian village...Ch. 18 - At what temperature is the Fahrenheit scale...Ch. 18 - On a linear X temperature scale, water freezes at...Ch. 18 - ILW Suppose that on a linear temperature scale X,...Ch. 18 - At 20C, a brass cube has edge length 30 cm. What...Ch. 18 - ILW A circular hole in an aluminum plate is 2.725...Ch. 18 - An aluminum flagpole is 33 m high. By how much...Ch. 18 - Prob. 11PCh. 18 - An aluminum-alloy rod has a length of 10.000 cm at...Ch. 18 - SSM Find the change in volume of an aluminum...Ch. 18 - When the temperature of a copper coin is raised by...Ch. 18 - ILW A steel rod is 3.000 cm in diameter at 25.00C....Ch. 18 - When the temperature of a metal cylinder is raised...Ch. 18 - SSM WWW An aluminum cup of 100 cm3 capacity is...Ch. 18 - At 20C, a rod is exactly 20.05 cm long on a steel...Ch. 18 - GO A vertical glass tube of length L = 1.280 000 m...Ch. 18 - GO In a certain experiment, a small radioactive...Ch. 18 - SSM ILW As a result of a temperature rise of 32 C,...Ch. 18 - One way to keep the contents of a garage from...Ch. 18 - SSM A small electric immersion healer is used to...Ch. 18 - A certain substance has a mass per mole of 50.0...Ch. 18 - Prob. 25PCh. 18 - What muss of butter, which has a usable energy...Ch. 18 - SSM Calculate the minimum amount of energy, in...Ch. 18 - How much water remains unfrozen after 50.2 kJ is...Ch. 18 - In a solar water heater, energy from the Sun is...Ch. 18 - A 0.400 kg simple is placed in a cooling apparatus...Ch. 18 - ILW What mass of steam at 100C must be mixed with...Ch. 18 - The specific heat of a substance varies with...Ch. 18 - Nonmetric version: a How long does a 2.0 105...Ch. 18 - GO Samples A and B are at different initial...Ch. 18 - An insulated Thermos contains l30 cm3 of hot...Ch. 18 - A 150 g copper bowl contains 220 g of water, both...Ch. 18 - A person makes a quantity of iced tea by mixing...Ch. 18 - A 0.530 kg sample of liquid water and a sample of...Ch. 18 - GO Ethyl alcohol has a boiling point of 78.0C, a...Ch. 18 - GO Calculate the specific heat of a metal from the...Ch. 18 - SSM WWW a Two 50 g ice cubes are dropped into 200...Ch. 18 - GO A 20.0 g copper ring at 0.000C has an inner...Ch. 18 - In Fig. 18-37, a gas sample expands from V0 to...Ch. 18 - GO A thermodynamic system is taken from stale A to...Ch. 18 - SSM ILW A gas within a closed chamber undergoes...Ch. 18 - Suppose 200 J of work is done on a system and 70.0...Ch. 18 - Prob. 47PCh. 18 - GO As a gas is held within a closed chamber, it...Ch. 18 - GO Figure 18-42 represents a closed cycle for a...Ch. 18 - GO A lab sample of gas is taken through cycle abca...Ch. 18 - A sphere of radius 0.500 m, temperature 27.0C, and...Ch. 18 - The ceiling of a single-family dwelling in a cold...Ch. 18 - SSM Consider the slab shown in Fig. 18-18. Suppose...Ch. 18 - If you were to walk briefly in space without a...Ch. 18 - ILW A cylindrical copper rod of length 1.2 m and...Ch. 18 - The giant hornet Vespa mandarinia japonica preys...Ch. 18 - Prob. 57PCh. 18 - A solid cylinder of radius r1 = 2.5 cm, length h1...Ch. 18 - Prob. 59PCh. 18 - GO Figure 18-46 shows the cross section of a wall...Ch. 18 - SSM A 5.0 cm slap has formed on an outdoor tank of...Ch. 18 - Leidenfrost effect. A water drop will last about 1...Ch. 18 - GO Figure 18-49 shows in cross section a wall...Ch. 18 - Prob. 64PCh. 18 - Ice has formed on a shallow pond, and a shady...Ch. 18 - GO Evaporative cooling of beverages. A cold...Ch. 18 - In the extrusion of cold chocolate from a tube,...Ch. 18 - Prob. 68PCh. 18 - Figure 18-51 displays a closed cycle for a gas....Ch. 18 - In a certain solar house, energy from the Sun is...Ch. 18 - A 0.300 kg sample is placed in a cooling apparatus...Ch. 18 - The average rate at which energy is conducted...Ch. 18 - What is the volume increase of an aluminum cube...Ch. 18 - In a series of experiment, block B is to be placed...Ch. 18 - Figure 18-54 displays a dosed cycle for a gas....Ch. 18 - Three equal-length straight rods, of aluminum,...Ch. 18 - SSM The temperature of a 0.700 kg cube of ice is...Ch. 18 - GO Icicles. Liquid water coats an active growing...Ch. 18 - SSM A sample of gas expands from an initial...Ch. 18 - Figure 18-56a shows a cylinder containing gas and...Ch. 18 - SSM A sample of gas undergoes a transition from an...Ch. 18 - Prob. 82PCh. 18 - SSM The temperature of a Pyrex disk is changed...Ch. 18 - a Calculate the rate at which body heat is...Ch. 18 - SSM A 2.50 kg Jump of aluminum is heated to 92.0C...Ch. 18 - A glass window pane is exactly 20 cm by 30 cm at...Ch. 18 - A recruit can join the semi-secret 300 F club at...Ch. 18 - A steel rod at 25.0C is bolted at both ends and...Ch. 18 - An athlete needs to lose weight and decides to do...Ch. 18 - Soon after Earth was formed, heat released by the...Ch. 18 - Prob. 91PCh. 18 - A rectangular plate of glass initially has the...Ch. 18 - Suppose that you intercept 5.0 103 of the energy...Ch. 18 - A thermometer of mass 0.0550 kg and of specific...Ch. 18 - A sample of gas expands from V1 = 1.0 m3 and p1 =...Ch. 18 - Figure 18-59 shows a composite bar of length L =...Ch. 18 - On finding your stove out of order, you decide to...Ch. 18 - The p-V diagram in the Fig. 18-60 shows two paths...Ch. 18 - A cube of edge length 6.0 106 m, emissivity 0.75,...Ch. 18 - A flow calorimeter is a device used to measure the...Ch. 18 - An object of mass 6.00 kg falls through a height...Ch. 18 - The Pyrex glass mirror in a telescope has a...Ch. 18 - The area A of a rectangular plate is ab = 1.4 m2....Ch. 18 - Consider the liquid in a barometer whose...Ch. 18 - A pendulum clock with a pendulum made of brass is...Ch. 18 - Prob. 106PCh. 18 - Prob. 107PCh. 18 - A 1700 kg Buick moving at 83 km/h brakes to a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
16.
a. Calculate the standard free energy change as a pair of electrons is transferred from succinate to mole...
Biochemistry: Concepts and Connections (2nd Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Fill in the blanks: a. The wrist is also known as the _________ region. b. The arm is also known as the _______...
Human Anatomy & Physiology (2nd Edition)
How are photosynthesis and respiration related to each other?
Microbiology: Principles and Explorations
What are four functions of connective tissue?
Anatomy & Physiology (6th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Q: What is the direction of the magnetic field at point A, due to the current I in a wire, in each of the cases 1 to 6 shown below? Note: point A is in the plane of the page. ▪A I I ▪A (1) (2) ▪A • I (out of page) (3) ▪A I x I (into page) ▪A ▪A I (4) (5) (6)arrow_forwardA tennis ball is thrown into the air with initial speed vo=46 m/s and angle (theta) 38 degrees from the ground. Find the distance it travels (x) when it hits the ground.arrow_forwardProblem 04.08 (17 points). Answer the following questions related to the figure below. ථි R₁ www R₂ E R₁ www ли R₁ A Use Kirchhoff's laws to calculate the currents through each battery and resistor in terms of R1, R2, E1, & E2. B Given that all the resistances and EMFs have positive values, if E₁ > E2 and R₁ > R2, which direction is the current flowing through E₁? Through R₂? C If E1 E2 and R₁ > R2, which direction is the current flowing through E₁? Through R2?arrow_forward
- A 105- and a 45.0-Q resistor are connected in parallel. When this combination is connected across a battery, the current delivered by the battery is 0.268 A. When the 45.0-resistor is disconnected, the current from the battery drops to 0.0840 A. Determine (a) the emf and (b) the internal resistance of the battery. 10 R2 R₁ ww R₁ Emf 14 Emf Final circuit Initial circuitarrow_forwardA ball is shot at an angle of 60° with the ground. What should be the initial velocity of the ball so that it will go inside the ring 8 meters away and 3 meters high. Suppose that you want the ball to be scored exactly at the buzzer, determine the required time to throw and shoot the ball. Full solution and figure if there is.arrow_forwardCorrect answer please. I will upvote.arrow_forward
- Define operational amplifierarrow_forwardA bungee jumper plans to bungee jump from a bridge 64.0 m above the ground. He plans to use a uniform elastic cord, tied to a harness around his body, to stop his fall at a point 6.00 m above the water. Model his body as a particle and the cord as having negligible mass and obeying Hooke's law. In a preliminary test he finds that when hanging at rest from a 5.00 m length of the cord, his body weight stretches it by 1.55 m. He will drop from rest at the point where the top end of a longer section of the cord is attached to the bridge. (a) What length of cord should he use? Use subscripts 1 and 2 respectively to represent the 5.00 m test length and the actual jump length. Use Hooke's law F = KAL and the fact that the change in length AL for a given force is proportional the length L (AL = CL), to determine the force constant for the test case and for the jump case. Use conservation of mechanical energy to determine the length of the rope. m (b) What maximum acceleration will he…arrow_forward9 V 300 Ω www 100 Ω 200 Ω www 400 Ω 500 Ω www 600 Ω ww 700 Ω Figure 1: Circuit symbols for a variety of useful circuit elements Problem 04.07 (17 points). Answer the following questions related to the figure below. A What is the equivalent resistance of the network of resistors in the circuit below? B If the battery has an EMF of 9V and is considered as an ideal batter (internal resistance is zero), how much current flows through it in this circuit? C If the 9V EMF battery has an internal resistance of 2 2, would this current be larger or smaller? By how much? D In the ideal battery case, calculate the current through and the voltage across each resistor in the circuit.arrow_forward
- helparrow_forwardIf the block does reach point B, how far up the curved portion of the track does it reach, and if it does not, how far short of point B does the block come to a stop? (Enter your answer in m.)arrow_forwardTruck suspensions often have "helper springs" that engage at high loads. One such arrangement is a leaf spring with a helper coil spring mounted on the axle, as shown in the figure below. When the main leaf spring is compressed by distance yo, the helper spring engages and then helps to support any additional load. Suppose the leaf spring constant is 5.05 × 105 N/m, the helper spring constant is 3.50 × 105 N/m, and y = 0.500 m. Truck body yo Main leaf spring -"Helper" spring Axle (a) What is the compression of the leaf spring for a load of 6.00 × 105 N? Your response differs from the correct answer by more than 10%. Double check your calculations. m (b) How much work is done in compressing the springs? ☑ Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY