
Concept explainers
(a)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
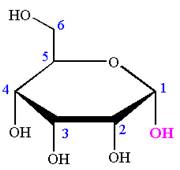
Explanation of Solution
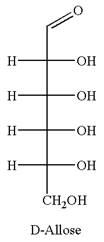
The given molecule is
The structure of
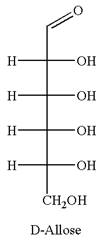
The name
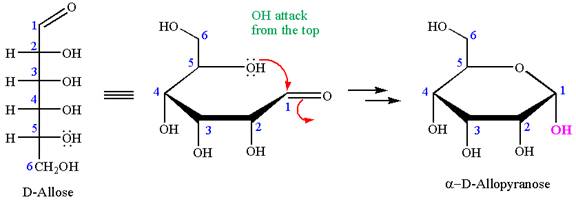
The Haworth projection of
(b)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
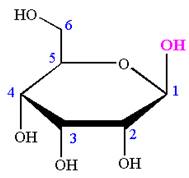
Explanation of Solution
The given molecule is
The structure of
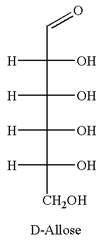
The name
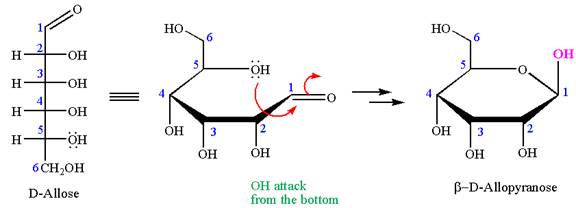
The Haworth projection of
(c)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
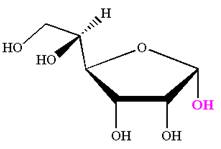
Explanation of Solution
The given molecule is
The structure of
The name
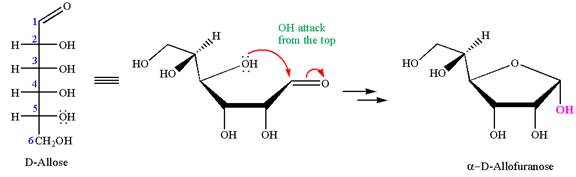
The Haworth projection of
(d)
Interpretation:
The Haworth projection for given molecule is to be drawn.
Concept introduction:
Structural drawings of carbohydrates in cyclic forms are called Haworth formulas. The
Answer to Problem 18.73P
The Haworth projection of
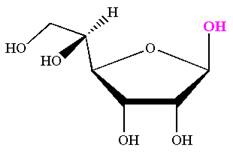
Explanation of Solution
The given molecule is
The structure of

The name
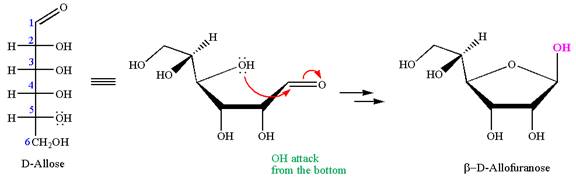
The Haworth projection of
Want to see more full solutions like this?
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

