
Concept explainers
(a)
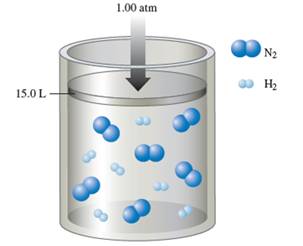
Interpretation: The partial pressure of ammonia in the container needs to be determined, when the reaction is complete at constant 1.00 atm and at constant volume.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(a)
Answer to Problem 126CP
Explanation of Solution
Number of
Number of
The balance chemical equation for the formation of ammonia:
Hence 1 molecule of
Hence number of
Thus remaining
Number of ammonia molecule formed = 2 x 2 = 4 molecules.
Total molecules at completion of reaction = 4 +4 = 16 molecules
Calculate mole fraction of ammonia:
Calculate partial pressure of
(b)
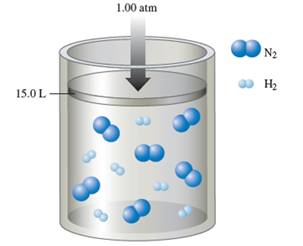
Interpretation: Interpret the mole fraction of ammonia in the container when the reaction is complete at constant 1.00 atm and at constant volume.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(b)
Answer to Problem 126CP
Explanation of Solution
Number of
Number of
The balance chemical equation for the formation of ammonia:
Hence 1 molecule of
Hence number of
Thus remaining
Number of ammonia molecule formed = 2 x 2 = 4 molecules.
Total molecules at completion of reaction = 4 +4 = 16 molecules
Calculate mole fraction of ammonia:
(c)
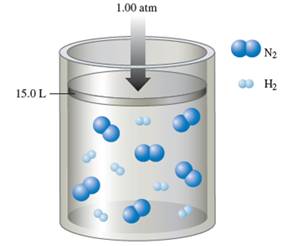
Interpretation:Interpret the volume of the container when the reaction complete if the initial pressure is 1.0 atm and volume is 15.0 L. The partial pressure of ammonia is 0.5 atm when reaction is complete.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(c)
Answer to Problem 126CP
Volume = 10.0 L
Explanation of Solution
Initial volume is 15 L. Now, total initial moles are 2n. The final number of moles can be calculated by taking sum of moles of ammonia and moles of nitrogen.
At constant temperature, pressure can be calculated for an ideal gas as follows:
Putting the values,
Thus, the final volume is 10 L.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemical Principles
- 6. Show how you would accomplish the following transformations. (Show the steps and reagents/solvents needed) 2-methylpropene →2,2-dimethyloxiran Iarrow_forward4) Answer the following exercise with curved arrows indicating who is a nucleophile or Who is the electrophile? 2.44 Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electron flow. 7 H3C OH N Pyridine Acetic acidarrow_forwardUsing the data provided please help me answer this question. Determine the concentration of the iron(Ill) salicylate in the unknown directly from to graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight line.arrow_forward
- Please help me figure out what the slope is and how to calculate the half life Using the data provided.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the structure of the missing reactants, intermediates, or products in the following mechanism. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. H Br2 (1 equiv) H- Select to Draw Starting Alkene Draw Major Product I I H2O 四: ⑦.. Q Draw Major Charged Intermediate Iarrow_forwardNH (aq)+CNO (aq) → CO(NH2)2(s) Experiment [NH4] (M) [CNO] (M) Initial rate (M/s) 1 0.014 0.02 0.002 23 0.028 0.02 0.008 0.014 0.01 0.001 Calculate the rate contant for this reaction using the data provided in the table.arrow_forward
- 2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forward
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





