
Concept explainers
(a)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(a)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
ion:
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
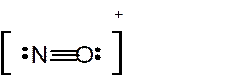
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(b)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(b)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
molecule :
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
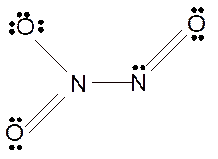
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(c)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(c)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
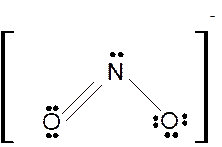
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(d)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(d)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
Number of valence electrons in N = 5
Total number of valence electrons in
Hence the Lewis structure of

The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemical Principles
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





