
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17.4, Problem 11P
Interpretation Introduction
Interpretation:
The compound which does not form an alcohol with excess Grignard reagent has to be identified.
Concept introduction:
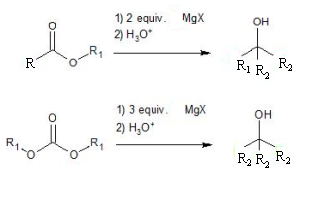
Reaction of
Reactions of Esters and Acyl Chlorides with Grignard reagents:
Ester and acyl chlorides undergoes nucleophilic acyl substitution and nucleophilic addition reaction when those are react with Grignard reagent.
Ester react with Grignard reagent
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or
neutral?
molecule
HO
H3N
+
The solution is...
X
O acidic
OH
O basic
H3N-CH-C-O
O neutral
○ (unknown)
O acidic
○ basic
CH2
CH 3-S-CH2
O neutral
○ (unknown)
H3N
O
OH
O acidic
O basic
Oneutral
O (unknown)
0
H3N-CH-C-O
CH3
CH
CH3
O acidic
O basic
O neutral
○ (unknown)
?
olo
Ar
BH
no Ai walkthroughs
need other product (product in picture is wrong dont submit the same thing)
How to solve this!
Chapter 17 Solutions
Organic Chemistry
Ch. 17.1 - Prob. 1PCh. 17.1 - Give two names for each of the following:Ch. 17.1 - Name the following:Ch. 17.2 - Prob. 4PCh. 17.4 - What products are formed when the following...Ch. 17.4 - We saw on the previous page that...Ch. 17.4 - a. How many stereoisomers are obtained from the...Ch. 17.4 - Prob. 9PCh. 17.4 - Write the mechanism for the reaction of acetyl...Ch. 17.4 - Prob. 11P
Ch. 17.4 - Show how the following compounds can be...Ch. 17.5 - Prob. 13PCh. 17.5 - Prob. 14PCh. 17.6 - In the mechanism for cyanohydrin formation, why is...Ch. 17.6 - Prob. 16PCh. 17.6 - Prob. 17PCh. 17.6 - Show two ways to convert an alkyl halide into a...Ch. 17.7 - Prob. 20PCh. 17.7 - Prob. 21PCh. 17.7 - Prob. 22PCh. 17.7 - Prob. 23PCh. 17.8 - Prob. 24PCh. 17.9 - What reducing agents should be used to obtain the...Ch. 17.9 - Prob. 26PCh. 17.9 - Prob. 27PCh. 17.10 - Prob. 28PCh. 17.10 - Prob. 29PCh. 17.10 - Prob. 30PCh. 17.10 - The pKa of protonated acetone is about 7.5. and...Ch. 17.10 - Prob. 32PCh. 17.10 - Prob. 33PCh. 17.10 - Prob. 34PCh. 17.10 - Excess ammonia must be used when a primary amine...Ch. 17.10 - The compounds commonly known as amino acids are...Ch. 17.11 - Hydration of an aldehyde is also catalyzed by...Ch. 17.11 - Which ketone forms the most hydrate in an aqueous...Ch. 17.11 - When trichloroacetaldehyde is dissolved in water,...Ch. 17.12 - Which of the following are a. hermiacetals? b....Ch. 17.12 - Prob. 41PCh. 17.12 - Explain why an acetal can be isolated but most...Ch. 17.13 - Prob. 43PCh. 17.13 - Prob. 44PCh. 17.13 - What products would be formed from the proceedings...Ch. 17.13 - a. In a six-step synthesis, what is the yield of...Ch. 17.13 - Show how each of the following compounds could be...Ch. 17.15 - Prob. 48PCh. 17.17 - Prob. 50PCh. 17.18 - Prob. 51PCh. 17.19 - Prob. 52PCh. 17 - Draw the structure for each of the following: a....Ch. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - a. Show the reagents required to form the primary...Ch. 17 - Prob. 57PCh. 17 - Using cyclohexanone as the starting material,...Ch. 17 - Prob. 59PCh. 17 - 60. Show how each of the following compounds could...Ch. 17 - Fill in the boxes:Ch. 17 - Prob. 62PCh. 17 - Identify A through O:Ch. 17 - Prob. 64PCh. 17 - Prob. 65PCh. 17 - Prob. 66PCh. 17 - How many signals would the product of the...Ch. 17 - Prob. 68PCh. 17 - Prob. 69PCh. 17 - Prob. 70PCh. 17 - Prob. 71PCh. 17 - Prob. 72PCh. 17 - Prob. 73PCh. 17 - Prob. 74PCh. 17 - Prob. 75PCh. 17 - Prob. 76PCh. 17 - Prob. 77PCh. 17 - A compound gives the following IR spectrum. Upon...Ch. 17 - a. Propose a mechanism for the following reaction:...Ch. 17 - Prob. 80PCh. 17 - Prob. 81PCh. 17 - Prob. 82PCh. 17 - Prob. 83PCh. 17 - Prob. 84PCh. 17 - In the presence of an acid catalyst, acetaldehyde...Ch. 17 - Prob. 86PCh. 17 - Prob. 87PCh. 17 - Prob. 88PCh. 17 - A compound reacts with methylmagnesium bromide...Ch. 17 - Show how each of the following compounds can be...Ch. 17 - Prob. 91PCh. 17 - Prob. 92PCh. 17 - The pKa values of the carboxylic acid groups of...Ch. 17 - The Baylis-Hillman reaction is a DABCO...Ch. 17 - Prob. 95PCh. 17 - Prob. 96P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I have a 2 mil plastic film that degrades in 22 days at 88C and 153 days at 61C what is the predicted theoretical degradation at 47C?arrow_forwardno ai walkthrougharrow_forwardI have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forward
- If a 5 film plastic film degraded in 30 days at 35C and the same film degraded in 10 days at 55 C and 2 days at 65C what would the predicted life time be at 22C for the same film?arrow_forwardno Ai walkthroughsarrow_forwardI have a aqueous solution (175 ml) of iridium trichloride containing 8,750 ppm Iridium by ICP OES analysis. What is the percent concentration of Iridium trichloride in aquous solution and provide the concentration in moles per liter, percentage by weight.arrow_forward
- no Ai walkthroughsarrow_forward136 PRACTICAL SPECTROSCOPY Compound 78 is a high-boiling liquid (boiling point 189° C) that contains halogen, but will not react with alkoxides to yield an halogen. ether. The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 35.32; H, 2.47; contains BC Spectral Data: doublet, 137.4 ppm; doublet, 130.1 ppm; doublet, 127.4 ppm; singlet, 97.3 ppm Absorbance Mass Spectrum Intensity 77 77 204 M + 128 40 60 80 100 120 140 160 180 m/e 200 220 280 240 260 300 Infrared Spectrum Wave Number, cm -1 4000 3000 2500 2000 1500 1300 1200 1100 1000 900 800 700 3 6 7 8 9 10 12 13 15 Wavelength, microns 'H NMR wwwww 5 Structure: www ppm, & ©2000 Brooks/Cole Publishing Com-arrow_forwardno Ai walkthroughsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning