
a.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
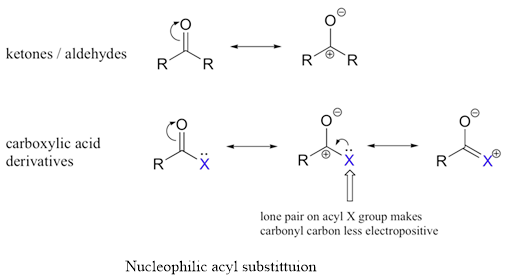
The carbonyl group of a
b.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

c.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

d.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

e.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

f.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

g.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

h.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

i.
Interpretation:
To write the products of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

j.
Interpretation:
To write the product of the given reaction.
Concept introduction:
Nucleophilic acyl substitution:
The carbonyl group of a carboxylic acid or carboxylic acid derivative is attached to a group that can be replaced with another group. Therefore, aldehyde and ketone undergoes nucleophilic acyl substitution reactions.

Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
Organic Chemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





