
(a)
Interpretation:
The
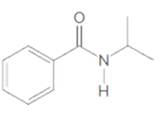
Concept Introduction:
(b)
Interpretation:
The carboxylic acid and amine that are needed to synthesis following amide should be determined:
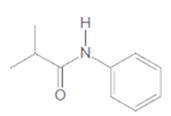
Concept Introduction:
Functional groups are the groups of atoms or atoms which are bonded with parent carbon chain in the organic molecule and are responsible for the physical and chemical properties of the compound. In organic chemistry, there are different functional groups such as carboxylic acid, alcohol, ester, or amide.
Amines are the organic compounds with general chemical formula of R-NH2 or R-NH-R whereas carboxylic acids are the organic molecules with R-COOH as general chemical formula.
Want to see the full answer?
Check out a sample textbook solution
Chapter 17 Solutions
CONNECT IA GENERAL ORGANIC&BIO CHEMISTRY
- Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes. H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)arrow_forwardProblem 5-31 Which of the following objects are chiral? (a) A basketball (d) A golf club (b) A fork (c) A wine glass (e) A spiral staircase (f) A snowflake Problem 5-32 Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2,4-Dimethylheptane (b) 5-Ethyl-3,3-dimethylheptane (c) cis-1,4-Dichlorocyclohexane Problem 5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11Cl (c) An alkene, C6H12 (b) An alcohol, C6H140 (d) An alkane, C8H18 Problem 5-36 Erythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. How H3C CH3 many chirality centers does erythronolide B have? OH Identify them. H3C -CH3 OH Erythronolide B H3C. H3C. OH OH CH3arrow_forwardPLEASE HELP! URGENT! PLEASE RESPOND!arrow_forward
- 2. Propose a mechanism for this reaction. ہلی سے ملی N H (excess)arrow_forwardSteps and explanationn please.arrow_forwardProblem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole





